Long-Term Tolerability and Efficacy of Single-Pill Combinations of Telmisartan 40-80 mg Plus Amlodipine 5 or 10 mg in Patients Whose Blood Pressure Was Not Initially Controlled by Amlodipine 5-10 mg: Open-Label, Long-Term Follow-Ups of the TEAMSTA-5 and TEAMSTA-10 Studies
- PMID: 24653513
- PMCID: PMC3954024
- DOI: 10.1016/j.curtheres.2012.02.004
Long-Term Tolerability and Efficacy of Single-Pill Combinations of Telmisartan 40-80 mg Plus Amlodipine 5 or 10 mg in Patients Whose Blood Pressure Was Not Initially Controlled by Amlodipine 5-10 mg: Open-Label, Long-Term Follow-Ups of the TEAMSTA-5 and TEAMSTA-10 Studies
Abstract
Background: Two 8-week, randomized, double-blind, controlled studies previously evaluated the efficacy and tolerability of single-pill combinations of telmisartan 40-80 mg/amlodipine 5-10 mg (T40-80/A5-10) in patients with hypertension not at diastolic blood pressure (DBP) goal (DBP <90 mm Hg) after 6 weeks of amlodipine 5 mg monotherapy (A5) (TEAMSTA-5) or amlodipine 10 mg monotherapy (A10) (TEAMSTA-10). The long-term (≥6 months) tolerability and efficacy of single-pill combinations of T40-T80/A5-A10 have now been evaluated in 2 open-label studies in patients who had successfully completed either TEAMSTA-5 or TEAMSTA-10 (TEAMSTA-5 and TEAMSTA-10 Follow-Ups).
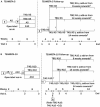
Methods: In the TEAMSTA-5 Follow-Up, 976 patients whose blood pressure was not initially controlled by taking A5 received T40/A5 for 4 or 8 weeks, with consecutive uptitration to T80/A5 if DBP was ≥90 mm Hg. In TEAMSTA-10 Follow-Up, 838 patients not initially achieving blood pressure control using A10 received T40/A10 for 4 weeks before randomization to T40/A10 or T80/A10; after 4 weeks, patients randomized to T40/A10 with DBP ≥90 mm Hg were uptitrated to T80/A10. In both studies, add-on antihypertensive medication was allowed if DBP was not at goal.
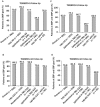
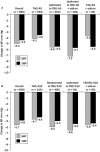
Results: Treatment compliance in both follow-up studies was ≥98.4%. Single-pill combinations of T40-T80/A5-A10 resulted in additional clinically relevant blood pressure reductions and 67% to 93% of patients achieved DBP goal (<90 mm Hg); only 1% to 19% of patients received additional medication for hypertension, of whom 29% to 76% achieved DBP goal. Long-term treatment with T40-T80/A5-A10 was well tolerated, with comparable adverse event profiles for all telmisartan/amlodipine combinations. The most common drug-related adverse events were peripheral edema (1.9%-3.9%) and dizziness (1.5% in the T80/A5 group only); these were consistent with the known tolerability profiles of telmisartan/amlodipine combinations. Overall treatment discontinuation rates due to adverse events were low (0.7%-1.5%).
Conclusions: In patients not achieving DBP goal with either A5 or A10 monotherapy, the vast majority achieved DBP goal with single-pill combinations of T40-T80/A5-A10. Long-term treatment was well tolerated with high compliance, promoting treatment adherence regardless of telmisartan/amlodipine dose. ClinicalTrials.gov identifiers: NCT00614380 (TEAMSTA-5 Follow-up) and NCT00624052 (TEAMSTA-10 Follow-up).
Keywords: amlodipine; angiotensin II receptor blocker; calcium channel blocker; essential hypertension; single-pill combination; telmisartan.
Figures

Similar articles
-
Switching patients with uncontrolled hypertension on amlodipine 10 mg to single-pill combinations of telmisartan and amlodipine: results of the TEAMSTA-10 study.Curr Med Res Opin. 2011 Nov;27(11):2145-53. doi: 10.1185/03007995.2011.624089. Epub 2011 Sep 29. Curr Med Res Opin. 2011. PMID: 21955225 Clinical Trial.
-
Telmisartan and amlodipine single-pill combinations vs amlodipine monotherapy for superior blood pressure lowering and improved tolerability in patients with uncontrolled hypertension: results of the TEAMSTA-5 study.J Clin Hypertens (Greenwich). 2011 Jul;13(7):459-66. doi: 10.1111/j.1751-7176.2011.00468.x. Epub 2011 Apr 22. J Clin Hypertens (Greenwich). 2011. PMID: 21762357 Free PMC article. Clinical Trial.
-
Comparison of fixed-dose combinations of telmisartan/hydrochlorothiazide 40/12.5 mg and 80/12.5 mg and a fixed-dose combination of losartan/hydrochlorothiazide 50/12.5 mg in mild to moderate essential hypertension: pooled analysis of two multicenter, prospective, randomized, open-label, blinded-end point (PROBE) trials.Clin Ther. 2005 Nov;27(11):1795-805. doi: 10.1016/j.clinthera.2005.11.014. Clin Ther. 2005. PMID: 16368450
-
Review: a single-pill combination of telmisartan plus amlodipine for the treatment of hypertension.Postgrad Med. 2011 Nov;123(6):58-65. doi: 10.3810/pgm.2011.11.2495. Postgrad Med. 2011. PMID: 22104454 Review.
-
Telmisartan/amlodipine: single-pill combination in hypertension.Am J Cardiovasc Drugs. 2010;10(6):401-12. doi: 10.2165/11204880-000000000-00000. Am J Cardiovasc Drugs. 2010. PMID: 21090832 Review.
Cited by
-
Long-term safety and efficacy of telmisartan/amlodipine single pill combination in the treatment of hypertension.Vasc Health Risk Manag. 2013;9:95-104. doi: 10.2147/VHRM.S40963. Epub 2013 Mar 16. Vasc Health Risk Manag. 2013. PMID: 23662062 Free PMC article. Review.
-
Clinical data analysis of telmisartan for hypertension management in Indian population.Bioinformation. 2021 Jun 30;17(6):652-659. doi: 10.6026/97320630017652. eCollection 2021. Bioinformation. 2021. PMID: 35173388 Free PMC article.
-
Fixed-dose combination therapy for the prevention of cardiovascular disease.Cochrane Database Syst Rev. 2014 Apr 16;4(4):CD009868. doi: 10.1002/14651858.CD009868.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2017 Mar 06;3:CD009868. doi: 10.1002/14651858.CD009868.pub3. PMID: 24737108 Free PMC article. Updated.
References
-
- Mancia G., Laurent S., Agabiti-Rosei E. Reappraisal of European guidelines on hypertension management: a European Society of Hypertension Task Force document. J Hypertens. 2009;27:2121–2158. - PubMed
-
- World Health Organization, International Society of Hypertension Writing Group 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. - PubMed
-
- Chobanian A.V., Bakris G.L., Black H.R. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. - PubMed
-
- Graham I., Atar D., Borch-Johnsen K. European guidelines on cardiovascular disease prevention in clinical practice. Eur J Cardiovasc Prev Rehabil. 2007;14(Suppl 2):S1–S113. - PubMed
-
- Mancia G., De Backer G., Dominiczak A. 2007 ESH–ESC practice guidelines for the management of arterial hypertension. J Hypertens. 2007;25:1105–1187. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

