Differential effects of cyclosporin and etanercept treatment on various pathologic parameters in a murine model of irradiation-induced mucositis
- PMID: 24653517
- PMCID: PMC3954008
- DOI: 10.1016/j.curtheres.2012.06.002
Differential effects of cyclosporin and etanercept treatment on various pathologic parameters in a murine model of irradiation-induced mucositis
Abstract
Background: Radiation therapy is the most prescribed treatment for many oncologic indications. One of its common side effects is mucositis with hallmark apoptosis in the intestinal crypt and diarrhea.
Objective: We investigated the potential beneficial effects of etanercept and cyclosporin treatment during radiation exposure. The effects of these drugs on intestinal apoptosis, long-term weight loss, diarrhea severity, and survival were examined.
Methods: For acute observation studies, animals pretreated with phosphate buffer saline (PBS) vehicle, either etanercept, or cyclosporin were challenged with either 1 Gy or 13 Gy irradiation and sacrificed 6 hours later. The animals' small intestines were then harvested for histologic analysis. For chronic survival studies, 14.5 Gy irradiation was applied. Etanercept or cyclosporin treatments were given 15 minutes before the irradiation, followed by daily administration.
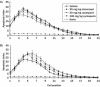
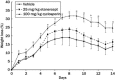
Results: At 6 hours postirradiation the maximum apoptotic index observed in the small intestine was ∼25% for both 1 Gy and 13 Gy irradiation. Etanercept and cyclosporin pretreatment had no effect on the irradiation-induced apoptosis. During chronic observation, the rate of weight loss was similar in all test groups. At 7 days postirradiation, the weight loss in phosphate buffered saline-treated control, etanercept, and cyclosporin groups reached a maximum at 19%, 24%, and 31.8%, respectively. The weight lost in the cyclosporin group was significantly higher than in the control group. Neither treatment reduced the severity of diarrhea, but cyclosporin increased the survival rate. Sixty percent of cyclosporin-treated animals survived compared with 27% in the PBS-treated control group and 47% in the etanercept-treated group. Serum tumor necrosis factor-α levels, a biomarker for both etanercept's mechanism of action and treatment efficacy, was inhibited by etanercept throughout the study, but cyclosporin only showed an inhibitory effect at 48 hours postirradiation.
Conclusions: Our study demonstrates that cyclosporin increases the survival rate of irradiated animals without affecting parameters such as intestinal histology, weight loss, and diarrhea severity.
Keywords: cyclosporin; etanercept; inflammation; irradiation; mucositis.
Figures




Similar articles
-
In vivo effects of immunomodulators in a murine model of Fluorouracil-induced mucositis.Curr Ther Res Clin Exp. 2011 Dec;72(6):262-72. doi: 10.1016/j.curtheres.2011.11.003. Curr Ther Res Clin Exp. 2011. PMID: 24648594 Free PMC article.
-
Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation.Int J Radiat Oncol Biol Phys. 2000 Jul 1;47(4):1033-42. doi: 10.1016/s0360-3016(00)00482-x. Int J Radiat Oncol Biol Phys. 2000. PMID: 10863076
-
Protective effect of genistein on radiation-induced intestinal injury in tumor bearing mice.BMC Complement Altern Med. 2013 May 14;13:103. doi: 10.1186/1472-6882-13-103. BMC Complement Altern Med. 2013. PMID: 23672582 Free PMC article.
-
A multi-component herbal preparation, STW 5, shows anti-apoptotic effects in radiation induced intestinal mucositis in rats.Phytomedicine. 2014 Sep 25;21(11):1390-9. doi: 10.1016/j.phymed.2014.04.030. Epub 2014 Jul 9. Phytomedicine. 2014. PMID: 25022208
-
Saireito (TJ-114), a Japanese traditional herbal medicine, reduces 5-fluorouracil-induced intestinal mucositis in mice by inhibiting cytokine-mediated apoptosis in intestinal crypt cells.PLoS One. 2015 Jan 7;10(1):e0116213. doi: 10.1371/journal.pone.0116213. eCollection 2015. PLoS One. 2015. PMID: 25565296 Free PMC article.
Cited by
-
Clinical development of new drug-radiotherapy combinations.Nat Rev Clin Oncol. 2016 Oct;13(10):627-42. doi: 10.1038/nrclinonc.2016.79. Epub 2016 Jun 1. Nat Rev Clin Oncol. 2016. PMID: 27245279
References
-
- Papa S., Bubici C., Zazzeroni F. The NF-kappaB-mediated control of the JNK cascade in the antagonism of programmed cell death in health and disease. Cell Death Diff. 2006;13:712–729. - PubMed
-
- Sonis S.T. Pathobiology of mucositis. Semin Oncol Nurs. 2004;20:11–15. - PubMed
-
- Pico J.L., Avila-Garavito A., Naccache P. Mucositis: its occurrence, consequences, and treatment in the oncology setting. Oncologist. 1998;3:446–451. - PubMed
-
- Logan R.M., Gibson R.J., Bowen J.M. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: implications for the pathobiology of mucositis. Cancer Chemother Pharmacol. 2008;62:33–41. - PubMed
-
- Sonis S.T. Pathobiology of oral mucositis: novel insights and opportunities. J Support Oncol. 2007;5:3–11. - PubMed
LinkOut - more resources
Full Text Sources

