Interrelation between protein synthesis, proteostasis and life span
- PMID: 24653664
- PMCID: PMC3958960
- DOI: 10.2174/1389202915666140210210542
Interrelation between protein synthesis, proteostasis and life span
Abstract
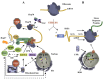
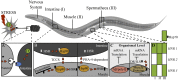
The production of newly synthesized proteins is a key process of protein homeostasis that initiates the biosynthetic flux of proteins and thereby determines the composition, stability and functionality of the proteome. Protein synthesis is highly regulated on multiple levels to adapt the proteome to environmental and physiological challenges such as aging and proteotoxic conditions. Imbalances of protein folding conditions are sensed by the cell that then trigger a cascade of signaling pathways aiming to restore the protein folding equilibrium. One regulatory node to rebalance proteostasis upon stress is the control of protein synthesis itself. Translation is reduced as an immediate response to perturbations of the protein folding equilibrium that can be observed in the cytosol as well as in the organelles such as the endoplasmatic reticulum and mitochondria. As reduction of protein synthesis is linked to life span increase, the signaling pathways regu-lating protein synthesis might be putative targets for treatments of age-related diseases. Eukaryotic cells have evolved a complex system for protein synthesis regulation and this review will summarize cellular strategies to regulate mRNA translation upon stress and its impact on longevity.
Keywords: Aging; Chaperone; Life span; Proteostasis; Stress response; UPR.; mRNA Translation.
Figures
References
-
- Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat. Rev. Mol. Cell Biol. 2005;6(4):318–327. - PubMed
-
- Ma S, Bhattacharjee R B, Bag J. Expression of poly(A)-binding protein is upregulated during recovery from heat shock in HeLa cells. FEBS J. 2009;276(2):552–570. - PubMed
-
- Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene. 2006;25(48):6384–6391. - PubMed
-
- Hansen M, Taubert S, Crawford D, Libina N, Lee S-J, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6(1):95–110. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
