Inhibitory glycinergic neurotransmission in the mammalian auditory brainstem upon prolonged stimulation: short-term plasticity and synaptic reliability
- PMID: 24653676
- PMCID: PMC3948056
- DOI: 10.3389/fncir.2014.00014
Inhibitory glycinergic neurotransmission in the mammalian auditory brainstem upon prolonged stimulation: short-term plasticity and synaptic reliability
Abstract
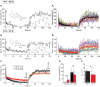
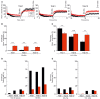
Short-term plasticity plays a key role in synaptic transmission and has been extensively investigated for excitatory synapses. Much less is known about inhibitory synapses. Here we analyze the performance of glycinergic connections between the medial nucleus of the trapezoid body (MNTB) and the lateral superior olive (LSO) in the auditory brainstem, where high spike rates as well as fast and precise neurotransmission are hallmarks. Analysis was performed in acute mouse slices shortly after hearing onset (postnatal day (P)11) and 8 days later (P19). Stimulation was done at 37°C with 1-400 Hz for 40 s. Moreover, in a novel approach named marathon experiments, a very prolonged stimulation protocol was employed, comprising 10 trials of 1-min challenge and 1-min recovery periods at 50 and 1 Hz, respectively, thus lasting up to 20 min and amounting to >30,000 stimulus pulses. IPSC peak amplitudes displayed short-term depression (STD) and synaptic attenuation in a frequency-dependent manner. No facilitation was observed. STD in the MNTB-LSO connections was less pronounced than reported in the upstream calyx of Held-MNTB connections. At P11, the STD level and the failure rate were slightly lower within the ms-to-s range than at P19. During prolonged stimulation periods lasting 40 s, P19 connections sustained virtually failure-free transmission up to frequencies of 100 Hz, whereas P11 connections did so only up to 50 Hz. In marathon experiments, P11 synapses recuperated reproducibly from synaptic attenuation during all recovery periods, demonstrating a robust synaptic machinery at hearing onset. At 26°C, transmission was severely impaired and comprised abnormally high amplitudes after minutes of silence, indicative of imprecisely regulated vesicle pools. Our study takes a fresh look at synaptic plasticity and stability by extending conventional stimulus periods in the ms-to-s range to minutes. It also provides a framework for future analyses of synaptic plasticity.
Keywords: fast-spiking cells; high-frequency neurotransmission; inhibitory postsynaptic currents; lateral superior olive; medial nucleus of the trapezoid body; short-term depression; synaptic attenuation; synaptic fidelity.
Figures











References
-
- Akaike N., Kaneda M. (1989). Glycine-gated chloride current in acutely isolated rat hypothalamic neurons. J. Neurophysiol. 62, 1400–1409 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

