ER bodies in plants of the Brassicales order: biogenesis and association with innate immunity
- PMID: 24653729
- PMCID: PMC3947992
- DOI: 10.3389/fpls.2014.00073
ER bodies in plants of the Brassicales order: biogenesis and association with innate immunity
Abstract
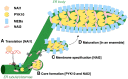
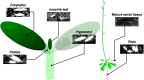
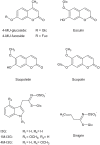
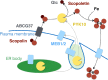
The endoplasmic reticulum (ER) forms highly organized network structures composed of tubules and cisternae. Many plant species develop additional ER-derived structures, most of which are specific for certain groups of species. In particular, a rod-shaped structure designated as the ER body is produced by plants of the Brassicales order, which includes Arabidopsis thaliana. Genetic analyses and characterization of A. thaliana mutants possessing a disorganized ER morphology or lacking ER bodies have provided insights into the highly organized mechanisms responsible for the formation of these unique ER structures. The accumulation of proteins specific for the ER body within the ER plays an important role in the formation of ER bodies. However, a mutant that exhibits morphological defects of both the ER and ER bodies has not been identified. This suggests that plants in the Brassicales order have evolved novel mechanisms for the development of this unique organelle, which are distinct from those used to maintain generic ER structures. In A. thaliana, ER bodies are ubiquitous in seedlings and roots, but rare in rosette leaves. Wounding of rosette leaves induces de novo formation of ER bodies, suggesting that these structures are associated with resistance against pathogens and/or herbivores. ER bodies accumulate a large amount of β-glucosidases, which can produce substances that potentially protect against invading pests. Biochemical studies have determined that the enzymatic activities of these β-glucosidases are enhanced during cell collapse. These results suggest that ER bodies are involved in plant immunity, although there is no direct evidence of this. In this review, we provide recent perspectives of ER and ER body formation in A. thaliana, and discuss clues for the functions of ER bodies. We highlight defense strategies against biotic stress that are unique for the Brassicales order, and discuss how ER structures could contribute to these strategies.
Keywords: ER body; endoplasmic reticulum; glucosinolate; organelle biogenesis; plant defenses; secondary metabolites; β-glucosidase.
Figures


Similar articles
-
Leaf Endoplasmic Reticulum Bodies Identified in Arabidopsis Rosette Leaves Are Involved in Defense against Herbivory.Plant Physiol. 2019 Apr;179(4):1515-1524. doi: 10.1104/pp.18.00984. Epub 2019 Jan 29. Plant Physiol. 2019. PMID: 30696747 Free PMC article.
-
ER Bodies Are Induced by Pseudomonas syringae and Negatively Regulate Immunity.Mol Plant Microbe Interact. 2021 Sep;34(9):1001-1009. doi: 10.1094/MPMI-11-20-0330-SC. Epub 2021 Sep 29. Mol Plant Microbe Interact. 2021. PMID: 34110257 Free PMC article.
-
PYK10 myrosinase reveals a functional coordination between endoplasmic reticulum bodies and glucosinolates in Arabidopsis thaliana.Plant J. 2017 Jan;89(2):204-220. doi: 10.1111/tpj.13377. Epub 2016 Dec 19. Plant J. 2017. PMID: 27612205
-
The ER body, a novel endoplasmic reticulum-derived structure in Arabidopsis.Plant Cell Physiol. 2003 Jul;44(7):661-6. doi: 10.1093/pcp/pcg089. Plant Cell Physiol. 2003. PMID: 12881493 Review.
-
Unique defense strategy by the endoplasmic reticulum body in plants.Plant Cell Physiol. 2011 Dec;52(12):2039-49. doi: 10.1093/pcp/pcr156. Epub 2011 Nov 18. Plant Cell Physiol. 2011. PMID: 22102697 Review.
Cited by
-
Large-Scale Transcriptome Analysis in Faba Bean (Vicia faba L.) under Ascochyta fabae Infection.PLoS One. 2015 Aug 12;10(8):e0135143. doi: 10.1371/journal.pone.0135143. eCollection 2015. PLoS One. 2015. PMID: 26267359 Free PMC article.
-
Specialist root herbivore modulates plant transcriptome and downregulates defensive secondary metabolites in a brassicaceous plant.New Phytol. 2022 Sep;235(6):2378-2392. doi: 10.1111/nph.18324. Epub 2022 Jul 9. New Phytol. 2022. PMID: 35717563 Free PMC article.
-
Endoplasmic reticulum-derived bodies enable a single-cell chemical defense in Brassicaceae plants.Commun Biol. 2020 Jan 14;3(1):21. doi: 10.1038/s42003-019-0739-1. Commun Biol. 2020. PMID: 31937912 Free PMC article.
-
MYC transcription factors coordinate tryptophan-dependent defence responses and compromise seed yield in Arabidopsis.New Phytol. 2022 Oct;236(1):132-145. doi: 10.1111/nph.18293. Epub 2022 Jun 21. New Phytol. 2022. PMID: 35642375 Free PMC article.
-
An unusual role for the phytyl chains in the photoprotection of the chlorophylls bound to Water-Soluble Chlorophyll-binding Proteins.Sci Rep. 2017 Aug 8;7(1):7504. doi: 10.1038/s41598-017-07874-6. Sci Rep. 2017. PMID: 28790428 Free PMC article.
References
-
- Adie B. A. T., Pérez-Pérez J., Pérez-Pérez M. M., Godoy M., Sánchez-Serrano J.-J., Schmelz E. A., et al. (2007). ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 19, 1665–1681 10.1105/tpc.106.048041 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

