Hepatitis C treatment & management
- PMID: 24653754
- PMCID: PMC3956092
Hepatitis C treatment & management
Abstract
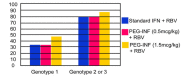
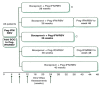
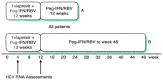
Combination therapy with pegylated interferon alfa (PEG-IFN alfa) and the nucleoside analogue ribavirin is the current standard of care in patients infected with hepatitis C virus (HCV). Patients with HCV genotype 1 have a much less favorable response to therapy and are treated for 12 months, compared with patients infected with genotypes 2 and 3, in whom a 6-month course of therapy is sufficient. If viremia is present after 6 months, additional therapy has a negligible benefit, and treatment should be stopped in all patients regardless of the viral genotype. With HIV coinfection, all patients with a response to therapy at the end of 6 months should receive an additional 6 months of combination therapy regardless of the genotype. Patients with acute HCV infection should be treated for 6 months. The addition of protease inhibitors to the combination of PEG-IFN alfa and ribavirin is becoming the new standard of care for the treatment of chronic HCV infection. Regimens that include a protease inhibitor significantly improve sustained virologic response rates in patients with genotype 1 HCV infection.
Keywords: hepatitis C; interferons; protease inhibitors; ribavirin.
Figures
References
-
- Dhawan VK. Hepatitis C. Treatment & Management. Updated. 2013
-
- Poynard T, Marcellin P, Lee SS. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet. 1998;352:1426–1432. - PubMed
-
- Manns MP, McHutchison JG, Gordon SC. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958–965. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. - PubMed
-
- Hadziyannis SJ, Sette H Jr, Morgan TR. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
