Neuroanatomical correlates of cognitive functioning in prodromal Huntington disease
- PMID: 24653952
- PMCID: PMC3937704
- DOI: 10.1002/brb3.185
Neuroanatomical correlates of cognitive functioning in prodromal Huntington disease
Abstract
Introduction: The brain mechanisms of cognitive impairment in prodromal Huntington disease (prHD) are not well understood. Although striatal atrophy correlates with some cognitive abilities, few studies of prHD have investigated whether cortical gray matter morphometry correlates in a regionally specific manner with functioning in different cognitive domains. This knowledge would inform the selection of cognitive measures for clinical trials that would be most sensitive to the target of a treatment intervention.
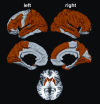
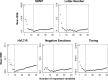
Method: In this study, random forest analysis was used to identify neuroanatomical correlates of functioning in five cognitive domains including attention and information processing speed, working memory, verbal learning and memory, negative emotion recognition, and temporal processing. Participants included 325 prHD individuals with varying levels of disease progression and 119 gene-negative controls with a family history of HD. In intermediate analyses, we identified brain regions that showed significant differences between the prHD and the control groups in cortical thickness and striatal volume. Brain morphometry in these regions was then correlated with cognitive functioning in each of the domains in the prHD group using random forest methods. We hypothesized that different regional patterns of brain morphometry would be associated with performances in distinct cognitive domains.
Results: The results showed that performances in different cognitive domains that are vulnerable to decline in prHD were correlated with regionally specific patterns of cortical and striatal morphometry. Putamen and/or caudate volumes were top-ranked correlates of performance across all cognitive domains, as was cortical thickness in regions related to the processing demands of each domain.
Conclusions: The results underscore the importance of identifying structural magnetic resonance imaging (sMRI) markers of functioning in different cognitive domains, as their relative sensitivity depends on the extent to which processing is called upon by different brain networks. The findings have implications for identifying neuroimaging and cognitive outcome measures for use in clinical trials.
Keywords: Cognition; magnetic resonance imaging; prodromal Huntington disease.
Figures


References
-
- Adolphs R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002;12:169–177. - PubMed
-
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. - PubMed
-
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends Cogn. Sci. 2008;12:193–200. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

