iPSC-derived forebrain neurons from FXS individuals show defects in initial neurite outgrowth
- PMID: 24654675
- PMCID: PMC4103262
- DOI: 10.1089/scd.2014.0030
iPSC-derived forebrain neurons from FXS individuals show defects in initial neurite outgrowth
Abstract
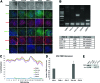
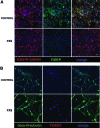
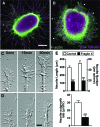
Fragile X syndrome (FXS) is the most common form of inherited intellectual disability and is closely linked with autism. The genetic basis of FXS is an expansion of CGG repeats in the 5'-untranslated region of the FMR1 gene on the X chromosome leading to the loss of expression of the fragile X mental retardation protein (FMRP). The cause of FXS has been known for over 20 years, yet the full molecular and cellular consequences of this mutation remain unclear. Although mouse and fly models have provided significant understanding of this disorder and its effects on the central nervous system, insight from human studies is limited. We have created human induced pluripotent stem cell (iPSC) lines from fibroblasts obtained from individuals with FXS to enable in vitro modeling of the human disease. Three young boys with FXS who came from a well-characterized cohort representative of the range of affectedness typical for the syndrome were recruited to aid in linking cellular and behavioral phenotypes. The FMR1 mutation is preserved during the reprogramming of patient fibroblasts to iPSCs. Mosaicism of the CGG repeat length in one of the patient's fibroblasts allowed for the generation of isogenic lines with differing CGG repeat lengths from the same patient. FXS forebrain neurons were differentiated from these iPSCs and display defective neurite initiation and extension. These cells provide a well-characterized resource to examine potential neuronal deficits caused by FXS as well as the function of FMRP in human neurons.
Figures

Similar articles
-
Epigenetic characterization of the FMR1 gene and aberrant neurodevelopment in human induced pluripotent stem cell models of fragile X syndrome.PLoS One. 2011;6(10):e26203. doi: 10.1371/journal.pone.0026203. Epub 2011 Oct 12. PLoS One. 2011. PMID: 22022567 Free PMC article.
-
CGG-repeat dynamics and FMR1 gene silencing in fragile X syndrome stem cells and stem cell-derived neurons.Mol Autism. 2016 Oct 6;7:42. doi: 10.1186/s13229-016-0105-9. eCollection 2016. Mol Autism. 2016. PMID: 27713816 Free PMC article.
-
Integrated transcriptome analysis of human iPS cells derived from a fragile X syndrome patient during neuronal differentiation.Sci China Life Sci. 2016 Nov;59(11):1093-1105. doi: 10.1007/s11427-016-0194-6. Epub 2016 Oct 11. Sci China Life Sci. 2016. PMID: 27730449
-
Human pluripotent stem cell models of Fragile X syndrome.Mol Cell Neurosci. 2016 Jun;73:43-51. doi: 10.1016/j.mcn.2015.11.011. Epub 2015 Nov 27. Mol Cell Neurosci. 2016. PMID: 26640241 Free PMC article. Review.
-
Across Dimensions: Developing 2D and 3D Human iPSC-Based Models of Fragile X Syndrome.Cells. 2022 May 24;11(11):1725. doi: 10.3390/cells11111725. Cells. 2022. PMID: 35681419 Free PMC article. Review.
Cited by
-
Novel fragile X syndrome 2D and 3D brain models based on human isogenic FMRP-KO iPSCs.Cell Death Dis. 2021 May 15;12(5):498. doi: 10.1038/s41419-021-03776-8. Cell Death Dis. 2021. PMID: 33993189 Free PMC article.
-
Transcriptome of iPSC-derived neuronal cells reveals a module of co-expressed genes consistently associated with autism spectrum disorder.Mol Psychiatry. 2021 May;26(5):1589-1605. doi: 10.1038/s41380-020-0669-9. Epub 2020 Feb 14. Mol Psychiatry. 2021. PMID: 32060413 Free PMC article.
-
Dysfunctional mTORC1 Signaling: A Convergent Mechanism between Syndromic and Nonsyndromic Forms of Autism Spectrum Disorder?Int J Mol Sci. 2017 Mar 18;18(3):659. doi: 10.3390/ijms18030659. Int J Mol Sci. 2017. PMID: 28335463 Free PMC article. Review.
-
FMRP Enhances the Translation of 4EBP2 mRNA during Neuronal Differentiation.Int J Mol Sci. 2023 Nov 14;24(22):16319. doi: 10.3390/ijms242216319. Int J Mol Sci. 2023. PMID: 38003508 Free PMC article.
-
FMRP regulates neurogenesis in vivo in Xenopus laevis tadpoles.eNeuro. 2015 Jan-Feb;2(1):e0055. doi: 10.1523/ENEURO.0055-14.2014. eNeuro. 2015. PMID: 25844398 Free PMC article.
References
-
- Hagerman RJ. and Hagerman PJ. (2002). Fragile X Syndrome. Johns Hopkins University Press, Baltimore MD
-
- Hagerman RJ, Ono MY. and Hagerman PJ. (2005). Recent advances in fragile X: a model for autism and neurodegeneration. Curr Opin Psychiatry 18:490–496 - PubMed
-
- Coffee B, Zhang F, Warren ST. and Reines D. (1999). Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat Genet 22:98–101 - PubMed
-
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, Reiner O, Richards S, Victoria MF. and Zhang FP. (1991). Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell 65:905–914 - PubMed
-
- Pieretti M, Zhang FP, Fu YH, Warren ST, Oostra BA, Caskey CT. and Nelson DL. (1991). Absence of expression of the FMR-1 gene in fragile X syndrome. Cell 66:817–822 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

