Multifunctional Fe₃O₄@polydopamine core-shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy
- PMID: 24654734
- PMCID: PMC4564054
- DOI: 10.1021/nn500722y
Multifunctional Fe₃O₄@polydopamine core-shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy
Abstract
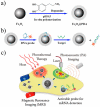
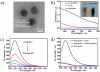
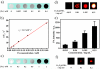
Multifunctional nanocomposites have the potential to integrate sensing, diagnostic, and therapeutic functions into a single nanostructure. Herein, we synthesize Fe3O4@polydopamine core-shell nanocomposites (Fe3O4@PDA NCs) through an in situ self-polymerization method. Dopamine, a melanin-like mimic of mussel adhesive proteins, can self-polymerize to form surface-adherent polydopamine (PDA) films onto a wide range of materials including Fe3O4 nanoparticles used here. In such nanocomposites, PDA provides a number of advantages, such as near-infrared absorption, high fluorescence quenching efficiency, and a surface for further functionalization with biomolecules. We demonstrate the ability of the Fe3O4@PDA NCs to act as theranostic agents for intracellular mRNA detection and multimodal imaging-guided photothermal therapy. This work would stimulate interest in the use of PDA as a useful material to construct multifunctional nanocomposites for biomedical applications.
Figures


Similar articles
-
Core-Shell-Shell Multifunctional Nanoplatform for Intracellular Tumor-Related mRNAs Imaging and Near-Infrared Light Triggered Photodynamic-Photothermal Synergistic Therapy.Anal Chem. 2017 Oct 3;89(19):10321-10328. doi: 10.1021/acs.analchem.7b02081. Epub 2017 Sep 13. Anal Chem. 2017. PMID: 28872842
-
EGFR-targeted delivery of DOX-loaded Fe3O4@ polydopamine multifunctional nanocomposites for MRI and antitumor chemo-photothermal therapy.Int J Nanomedicine. 2017 Apr 10;12:2899-2911. doi: 10.2147/IJN.S131418. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28435266 Free PMC article.
-
cRGD-Conjugated Fe3O4@PDA-DOX Multifunctional Nanocomposites for MRI and Antitumor Chemo-Photothermal Therapy.Int J Nanomedicine. 2019 Dec 5;14:9631-9645. doi: 10.2147/IJN.S222797. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31824156 Free PMC article.
-
Mussel-Inspired Polydopamine: The Bridge for Targeting Drug Delivery System and Synergistic Cancer Treatment.Macromol Biosci. 2020 Oct;20(10):e2000222. doi: 10.1002/mabi.202000222. Epub 2020 Aug 6. Macromol Biosci. 2020. PMID: 32761887 Review.
-
Polydopamine-Incorporated Nanoformulations for Biomedical Applications.Macromol Biosci. 2020 Dec;20(12):e2000228. doi: 10.1002/mabi.202000228. Epub 2020 Aug 23. Macromol Biosci. 2020. PMID: 32830435 Review.
Cited by
-
Engineering Polymeric Nanosystems against Oral Diseases.Molecules. 2021 Apr 13;26(8):2229. doi: 10.3390/molecules26082229. Molecules. 2021. PMID: 33924289 Free PMC article. Review.
-
Preparation of Multifunctional Dopamine-Coated Zerovalent Iron/Reduced Graphene Oxide for Targeted Phototheragnosis in Breast Cancer.Nanomaterials (Basel). 2020 Oct 1;10(10):1957. doi: 10.3390/nano10101957. Nanomaterials (Basel). 2020. PMID: 33019538 Free PMC article.
-
Polydopamine Surface Chemistry: A Decade of Discovery.ACS Appl Mater Interfaces. 2018 Mar 7;10(9):7523-7540. doi: 10.1021/acsami.7b19865. Epub 2018 Feb 26. ACS Appl Mater Interfaces. 2018. PMID: 29465221 Free PMC article.
-
Iron Oxide Nanoparticles in Photothermal Therapy.Molecules. 2018 Jun 28;23(7):1567. doi: 10.3390/molecules23071567. Molecules. 2018. PMID: 29958427 Free PMC article. Review.
-
Magnetic and near-infrared derived heating characteristics of dimercaptosuccinic acid coated uniform Fe@Fe3O4 core-shell nanoparticles.Nano Converg. 2020 Jun 8;7(1):20. doi: 10.1186/s40580-020-00229-4. Nano Converg. 2020. PMID: 32514813 Free PMC article.
References
-
- Lee JE, Lee N, Kim T, Kim J, Hyeon T. Multifunctional Mesoporous Silica Nanocomposite Nanoparticles for Theranostic Applications. Acc. Chem. Res. 2011;44:893–902. - PubMed
-
- Lee D-E, Koo H, Sun I-C, Ryu JH, Kim K, Kwon IC. Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev. 2012;41:2656–2672. - PubMed
-
- Gao J, Gu H, Xu B. Multifunctional Magnetic Nanoparticles: Design, Synthesis, and Biomedical Applications. Acc. Chem. Res. 2009;42:1097–1107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

