Metabolic regulation of immune responses
- PMID: 24655299
- PMCID: PMC5800786
- DOI: 10.1146/annurev-immunol-032713-120236
Metabolic regulation of immune responses
Abstract
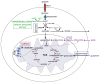
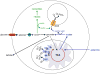
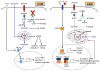
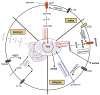
The immune system defends against pathogens and maintains tissue homeostasis for the life of the organism. These diverse functions are bioenergetically expensive, requiring precise control of cellular metabolic pathways. Although initial observations in this area were made almost a century ago, studies over the past decade have elucidated the molecular basis for how extracellular signals control the uptake and catabolism of nutrients in quiescent and activated immune cells. Collectively, these studies have revealed that the metabolic pathways of oxidative metabolism, glycolysis, and glutaminolysis preferentially fuel the cell fate decisions and effector functions of immune cells. Here, we discuss these findings and provide a general framework for understanding how metabolism fuels and regulates the maturation of immune responses. A better understanding of the metabolic checkpoints that control these transitions might provide new insights for modulating immunity in infection, cancer, or inflammatory disorders.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
References
-
- Taniguchi C, Emanuelli B, Kahn C. Critical nodes in signalling pathways: insights into insulin action. Nature reviews. Molecular cell biology. 2006;7:85–96. - PubMed
-
- Levene P, Meyer G. The action of leucocytes on glucose. Journal of Biological Chemistry 1912
-
- Newsholme E, Crabtree B, Ardawi M. Glutamine metabolism in lymphocytes: its biochemical, physiological and clinical importance. Quarterly journal of experimental physiology (Cambridge, England) 1985;70:473–89. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

