The FRET signatures of noninteracting proteins in membranes: simulations and experiments
- PMID: 24655506
- PMCID: PMC3984992
- DOI: 10.1016/j.bpj.2014.01.039
The FRET signatures of noninteracting proteins in membranes: simulations and experiments
Abstract
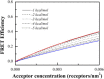
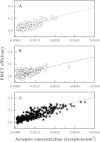
Förster resonance energy transfer (FRET) experiments are often used to study interactions between integral membrane proteins in cellular membranes. However, in addition to the FRET of sequence-specific interactions, these experiments invariably record a contribution due to proximity FRET, which occurs when a donor and an acceptor approach each other by chance within distances of ∼100 Å. This effect does not reflect specific interactions in the membrane and is frequently unappreciated, despite the fact that its magnitude can be significant. Here we develop a computational description of proximity FRET, simulating the cases of proximity FRET when fluorescent proteins are used to tag monomeric, dimeric, trimeric, and tetrameric membrane proteins, as well as membrane proteins existing in monomer-dimer equilibria. We also perform rigorous experimental measurements of this effect, by identifying membrane receptors that do not associate in mammalian membranes. We measure the FRET efficiencies between yellow fluorescent protein and mCherry-tagged versions of these receptors in plasma-membrane-derived vesicles as a function of receptor concentration. Finally, we demonstrate that the experimental measurements are well described by our predictions. The work presented here brings additional rigor to FRET-based studies of membrane protein interactions, and should have broad utility in membrane biophysics research.
Copyright © 2014 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


Comment in
-
Taking care of bystander FRET in a crowded cell membrane environment.Biophys J. 2014 Mar 18;106(6):1227-8. doi: 10.1016/j.bpj.2014.02.004. Biophys J. 2014. PMID: 24655495 Free PMC article. No abstract available.
-
Background FRET.Nat Methods. 2014 May;11(5):477. doi: 10.1038/nmeth.2954. Nat Methods. 2014. PMID: 24820373 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

