Design of co-crystals/salts of some Nitrogenous bases and some derivatives of thiophene carboxylic acids through a combination of hydrogen and halogen bonds
- PMID: 24655545
- PMCID: PMC3996520
- DOI: 10.1186/1752-153X-8-20
Design of co-crystals/salts of some Nitrogenous bases and some derivatives of thiophene carboxylic acids through a combination of hydrogen and halogen bonds
Abstract
Background: The utility of N-heterocyclic bases to obtain molecular complexes with carboxylic acids is well studied. Depending on the solid state interaction between the N-heterocyclic base and a carboxylic acid a variety of neutral or ionic synthons are observed. Meanwhile, pyridines and pyrimidines have been frequently chosen in the area of crystal engineering for their multipurpose functionality. HT (hetero trimers) and LHT (linear heterotetramers) are the well known synthons that are formed in the presence of pyrimidines and carboxylic acids.
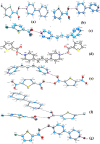
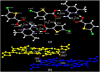
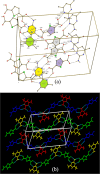
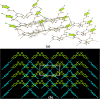
Results: Fourteen crystals involving various substituted thiophene carboxylic acid derivatives and nitrogenous bases were prepared and characterized by using single crystal X-ray diffraction. The 14 crystals can further be divided into two groups [1a-7a], [8b-14b] based on the nature of the nitrogenous base. Carboxylic acid to pyridine proton transfer has occurred in 3 compounds of each group. In addition to the commonly occurring hydrogen bond based pyridine/carboxylic acid and pyrimidine/carboxylic acid synthons which is the reason for assembly of primary motifs, various other interactions like Cl…Cl, Cl…O, C-H…Cl, C-H…S add additional support in organizing these supermolecules into extended architectures. It is also interesting to note that in all the compounds π-π stacking occurs between the pyrimidine-pyrimidine or pyridine-pyridine or acid-acid moieties rather than acid-pyrimidine/pyridine.
Conclusions: In all the compounds (1a-14b) either neutral O-H…Npyridyl/pyrimidine or charge-assisted Npyridinium-H…Ocarboxylate hydrogen bonds are present. The HT (hetero trimers) and LHT (linear heterotetramers) are dominant in the crystal structures of the adducts containing N-heterocyclic bases with two proton acceptors (1a-7a). Similar type supramolecular ladders are observed in 5TPC44BIPY (8b), TPC44BIPY (9b), TPC44TMBP (11b). Among the seven compounds [8b-14b] the extended ligands are linear in all except for the TMBP (10b, 11b, 12b). The structure of each compound depends on the dihedral angle between the carboxyl group and the nitrogenous base. All these compounds indicate three main synthons that regularly occur, namely linear heterodimer (HD), heterotrimer (HT) and heterotetramer (LHT).
Keywords: 5-chlorothiophen-2-carboxylic acid; Bipyridine; Cocrystal; Halogen bonding; Pyrimidine; Salts.
Figures

















References
-
- Desiraju GR, Steiner T. The Weak Hydrogen Bond: In Structural Chemistry and Biology. Vol. 9. New York: Oxford University Press; 2001.
-
- Jeffrey GA. An Introduction to Hydrogen Bonding, Volume 12. New York: Oxford University Press; 1997.
-
- Desiraju GR. Supramolecular synthons in crystal engineering—a new organic synthesis. Angew Chem Int Ed Engl. 1995;34:2311–2327.
-
- Schmidt GMJ. Photodimerization in the solid state. Pure Appl Chem. 1971;27:126.
-
- Almarsson O, Zaworotko MJ. Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines? Chem Commun. 2004;17:1889–1896. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

