Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection
- PMID: 24655914
- PMCID: PMC3997931
- DOI: 10.1186/1471-2148-14-56
Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection
Abstract
Background: Host defense against pathogenic infection is composed of resistance and tolerance. Resistance is the ability of the host to limit a pathogen burden, whereas tolerance is the ability to limit the deleterious effects of a given pathogen burden. This distinction recognizes that the fittest host does not necessarily have the most aggressive immune system, suggesting that host-pathogen co-evolution involves more than an escalating arms race between pathogen virulence factors and host antimicrobial activity. How a host balances resistance and tolerance and how this balance influences the evolution of host defense remains unanswered. In order to determine how genotype-by-diet interactions and evolutionary costs of each strategy may constrain the evolution of host defense, we measured survival, fecundity, and pathogen burden over five days in ten genotypes of Drosophila melanogaster reared on two diets and infected with the Gram-negative bacterial pathogen Providencia rettgeri.
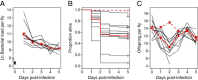
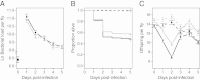
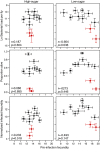
Results: We demonstrated two distinct phases of infection: an acute phase that consists of high mortality, low fecundity, and high pathogen loads, and a chronic phase where there was a substantial but stable pathogen load and mortality and fecundity returned to uninfected levels. We demonstrated genetic variation for resistance in both phases of infection, but found genetic variation for tolerance only in the acute phase. We found genotype-by-diet interactions for tolerance, especially in the acute phase, but genotype-by-diet interaction did not significantly shape resistance. We found a diet-dependent positive relationship between resistance and tolerance and a weak evolutionary cost of resistance, but did not detect any costs of tolerance.
Conclusions: Existing models of tolerance and resistance are overly simplistic. Multi-phase infections such as that studied here are rarely considered, but we show important differences in determination and evolutionary constraints on tolerance and resistance over the two phases of infection. Our observation of genetic variation for tolerance is inconsistent with simple models that predict evolutionary fixation of tolerance alleles, and instead indicate that genetic variation for resistance and tolerance is likely to be maintained by non-independence between resistance and tolerance, condition-dependent evolutionary costs, and environmental heterogeneity.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

