Atherosclerosis and Alzheimer--diseases with a common cause? Inflammation, oxysterols, vasculature
- PMID: 24656052
- PMCID: PMC3994432
- DOI: 10.1186/1471-2318-14-36
Atherosclerosis and Alzheimer--diseases with a common cause? Inflammation, oxysterols, vasculature
Abstract
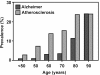
Background: Aging is accompanied by increasing vulnerability to pathologies such as atherosclerosis (ATH) and Alzheimer disease (AD). Are these different pathologies, or different presentations with a similar underlying pathoetiology?
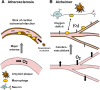
Discussion: Both ATH and AD involve inflammation, macrophage infiltration, and occlusion of the vasculature. Allelic variants in common genes including APOE predispose to both diseases. In both there is strong evidence of disease association with viral and bacterial pathogens including herpes simplex and Chlamydophila. Furthermore, ablation of components of the immune system (or of bone marrow-derived macrophages alone) in animal models restricts disease development in both cases, arguing that both are accentuated by inflammatory/immune pathways. We discuss that amyloid β, a distinguishing feature of AD, also plays a key role in ATH. Several drugs, at least in mouse models, are effective in preventing the development of both ATH and AD. Given similar age-dependence, genetic underpinnings, involvement of the vasculature, association with infection, Aβ involvement, the central role of macrophages, and drug overlap, we conclude that the two conditions reflect different manifestations of a common pathoetiology.
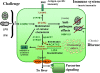
Mechanism: Infection and inflammation selectively induce the expression of cholesterol 25-hydroxylase (CH25H). Acutely, the production of 'immunosterol' 25-hydroxycholesterol (25OHC) defends against enveloped viruses. We present evidence that chronic macrophage CH25H upregulation leads to catalyzed esterification of sterols via 25OHC-driven allosteric activation of ACAT (acyl-CoA cholesterol acyltransferase/SOAT), intracellular accumulation of cholesteryl esters and lipid droplets, vascular occlusion, and overt disease.
Summary: We postulate that AD and ATH are both caused by chronic immunologic challenge that induces CH25H expression and protection against particular infectious agents, but at the expense of longer-term pathology.
Figures




Similar articles
-
ACAT1 gene ablation increases 24(S)-hydroxycholesterol content in the brain and ameliorates amyloid pathology in mice with AD.Proc Natl Acad Sci U S A. 2010 Feb 16;107(7):3081-6. doi: 10.1073/pnas.0913828107. Epub 2010 Jan 26. Proc Natl Acad Sci U S A. 2010. PMID: 20133765 Free PMC article.
-
Acyl-CoA:cholesterol acyltransferases (ACATs/SOATs): Enzymes with multiple sterols as substrates and as activators.J Steroid Biochem Mol Biol. 2015 Jul;151:102-7. doi: 10.1016/j.jsbmb.2014.09.008. Epub 2014 Sep 12. J Steroid Biochem Mol Biol. 2015. PMID: 25218443 Free PMC article. Review.
-
Blood-derived plasminogen drives brain inflammation and plaque deposition in a mouse model of Alzheimer's disease.Proc Natl Acad Sci U S A. 2018 Oct 9;115(41):E9687-E9696. doi: 10.1073/pnas.1811172115. Epub 2018 Sep 25. Proc Natl Acad Sci U S A. 2018. PMID: 30254165 Free PMC article.
-
Does the oxysterol 27-hydroxycholesterol underlie Alzheimer's disease-Parkinson's disease overlap?Exp Gerontol. 2015 Aug;68:13-8. doi: 10.1016/j.exger.2014.09.013. Epub 2014 Sep 28. Exp Gerontol. 2015. PMID: 25261765 Free PMC article. Review.
-
Role of acyl-coenzyme a: cholesterol acyltransferase activity in the processing of the amyloid precursor protein.J Mol Neurosci. 2004;24(1):93-6. doi: 10.1385/JMN:24:1:093. J Mol Neurosci. 2004. PMID: 15314256 Review.
Cited by
-
On entropy and information in gene interaction networks.Bioinformatics. 2019 Mar 1;35(5):815-822. doi: 10.1093/bioinformatics/bty691. Bioinformatics. 2019. PMID: 30102349 Free PMC article.
-
FMNL2 regulates gliovascular interactions and is associated with vascular risk factors and cerebrovascular pathology in Alzheimer's disease.Acta Neuropathol. 2022 Jul;144(1):59-79. doi: 10.1007/s00401-022-02431-6. Epub 2022 May 24. Acta Neuropathol. 2022. PMID: 35608697 Free PMC article.
-
Cerebrovascular Changes and Neurodegeneration Related to Hyperlipidemia: Characteristics of the Human ApoB-100 Transgenic Mice.Curr Pharm Des. 2020;26(13):1486-1494. doi: 10.2174/1381612826666200218101818. Curr Pharm Des. 2020. PMID: 32067608 Free PMC article.
-
Microbes and Alzheimer's Disease.J Alzheimers Dis. 2016;51(4):979-84. doi: 10.3233/JAD-160152. J Alzheimers Dis. 2016. PMID: 26967229 Free PMC article. Review. No abstract available.
-
Effects of acute pro-inflammatory stimulation and 25-hydroxycholesterol on hippocampal plasticity and learning involve NLRP3 inflammasome and cellular stress responses.Sci Rep. 2025 Feb 20;15(1):6149. doi: 10.1038/s41598-025-90149-2. Sci Rep. 2025. PMID: 39979396 Free PMC article.
References
-
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS. et al. Heart disease and stroke statistics–2011 update: a report from the American Heart Association. Circulation. 2011;14:e18–e209. doi: 10.1161/CIR.0b013e3182009701. - DOI - PMC - PubMed
-
- Lim LS, Haq N, Mahmood S, Hoeksema L. Atherosclerotic cardiovascular disease screening in adults: American College Of Preventive Medicine position statement on preventive practice. Am J Prev Med. 2011;14:381–10. - PubMed
-
- Chan KY, Wang W, Wu JJ, Liu L, Theodoratou E, Car J, Middleton L, Russ TC, Deary IJ, Campbell H, Wang W, Rudan I. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet. 2013;14:2016–2023. doi: 10.1016/S0140-6736(13)60221-4. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

