Prostaglandin E2 alters Wnt-dependent migration and proliferation in neuroectodermal stem cells: implications for autism spectrum disorders
- PMID: 24656144
- PMCID: PMC4233645
- DOI: 10.1186/1478-811X-12-19
Prostaglandin E2 alters Wnt-dependent migration and proliferation in neuroectodermal stem cells: implications for autism spectrum disorders
Abstract
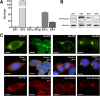
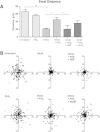
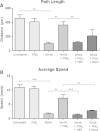
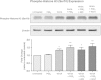
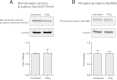
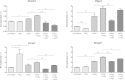
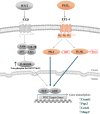
Prostaglandin E2 (PGE2) is a natural lipid-derived molecule that is involved in important physiological functions. Abnormal PGE2 signalling has been associated with pathologies of the nervous system. Previous studies provide evidence for the interaction of PGE2 and canonical Wnt signalling pathways in non-neuronal cells. Since the Wnt pathway is crucial in the development and organization of the brain, the main goal of this study is to determine whether collaboration between these pathways exists in neuronal cell types. We report that PGE2 interacts with canonical Wnt signalling through PKA and PI-3K in neuroectodermal (NE-4C) stem cells. We used time-lapse microscopy to determine that PGE2 increases the final distance from origin, path length travelled, and the average speed of migration in Wnt-activated cells. Furthermore, PGE2 alters distinct cellular phenotypes that are characteristic of Wnt-induced NE-4C cells, which corresponds to the modified splitting behaviour of the cells. We also found that in Wnt-induced cells the level of β-catenin protein was increased and the expression levels of Wnt-target genes (Ctnnb1, Ptgs2, Ccnd1, Mmp9) was significantly upregulated in response to PGE2 treatment. This confirms that PGE2 activated the canonical Wnt signalling pathway. Furthermore, the upregulated genes have been previously associated with ASD. Our findings show, for the first time, evidence for cross-talk between PGE2 and Wnt signalling in neuronal cells, where PKA and PI-3K might act as mediators between the two pathways. Given the importance of PGE2 and Wnt signalling in prenatal development of the nervous system, our study provides insight into how interaction between these two pathways may influence neurodevelopment.
Figures


Similar articles
-
Prostaglandin E2 promotes neural proliferation and differentiation and regulates Wnt target gene expression.J Neurosci Res. 2016 Aug;94(8):759-75. doi: 10.1002/jnr.23759. Epub 2016 Jun 5. J Neurosci Res. 2016. PMID: 27265882
-
Prostaglandin E2 elevates calcium in differentiated neuroectodermal stem cells.Mol Cell Neurosci. 2016 Jul;74:71-7. doi: 10.1016/j.mcn.2016.03.010. Epub 2016 Apr 10. Mol Cell Neurosci. 2016. PMID: 27074429
-
Microarray analysis of gene expression in the cyclooxygenase knockout mice - a connection to autism spectrum disorder.Eur J Neurosci. 2018 Mar;47(6):750-766. doi: 10.1111/ejn.13781. Epub 2017 Dec 15. Eur J Neurosci. 2018. PMID: 29161772
-
Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways.Cancer Sci. 2016 Apr;107(4):391-7. doi: 10.1111/cas.12901. Epub 2016 Mar 18. Cancer Sci. 2016. PMID: 27079437 Free PMC article. Review.
-
The PTGS2/COX2-PGE2 signaling cascade in inflammation: Pro or anti? A case study with type 1 diabetes mellitus.Int J Biol Sci. 2023 Aug 6;19(13):4157-4165. doi: 10.7150/ijbs.86492. eCollection 2023. Int J Biol Sci. 2023. PMID: 37705740 Free PMC article. Review.
Cited by
-
An in vivo drug screen in zebrafish reveals that cyclooxygenase 2-derived prostaglandin D2 promotes spinal cord neurogenesis.Cell Prolif. 2024 May;57(5):e13594. doi: 10.1111/cpr.13594. Epub 2023 Dec 28. Cell Prolif. 2024. PMID: 38155412 Free PMC article.
-
Lipid Peroxidation via Regulating the Metabolism of Docosahexaenoic Acid and Arachidonic Acid in Autistic Behavioral Symptoms.Curr Issues Mol Biol. 2023 Nov 15;45(11):9149-9164. doi: 10.3390/cimb45110574. Curr Issues Mol Biol. 2023. PMID: 37998751 Free PMC article.
-
Lipid-Based Molecules on Signaling Pathways in Autism Spectrum Disorder.Int J Mol Sci. 2022 Aug 29;23(17):9803. doi: 10.3390/ijms23179803. Int J Mol Sci. 2022. PMID: 36077195 Free PMC article. Review.
-
Lipid Peroxidation With Implication of Organic Pollution in Autistic Behaviors.Cureus. 2021 Mar 30;13(3):e14188. doi: 10.7759/cureus.14188. Cureus. 2021. PMID: 33936898 Free PMC article.
-
Neurodevelopmental Perspectives on Wnt Signaling in Psychiatry.Mol Neuropsychiatry. 2017 Feb;2(4):219-246. doi: 10.1159/000453266. Epub 2017 Jan 13. Mol Neuropsychiatry. 2017. PMID: 28277568 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

