Multisensory integration: flexible use of general operations
- PMID: 24656248
- PMCID: PMC4090761
- DOI: 10.1016/j.neuron.2014.02.044
Multisensory integration: flexible use of general operations
Abstract
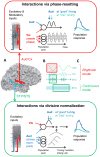
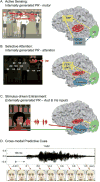
Research into the anatomical substrates and "principles" for integrating inputs from separate sensory surfaces has yielded divergent findings. This suggests that multisensory integration is flexible and context dependent and underlines the need for dynamically adaptive neuronal integration mechanisms. We propose that flexible multisensory integration can be explained by a combination of canonical, population-level integrative operations, such as oscillatory phase resetting and divisive normalization. These canonical operations subsume multisensory integration into a fundamental set of principles as to how the brain integrates all sorts of information, and they are being used proactively and adaptively. We illustrate this proposition by unifying recent findings from different research themes such as timing, behavioral goal, and experience-related differences in integration.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Alvarado J, Vaughan J, Stanford T, Stein B. Multisensory versus unisensory integration: contrasting modes in the superior colliculus. Journal of Neurophysiology. 2007;97:3193–3205. - PubMed
-
- Bach-y-Rita P, W Kercel S. Sensory substitution and the human-machine interface. Trends in Cognitive Sciences. 2003;7:541–546. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

