Enhancement of inhibitory neurotransmission by GABAA receptors having α2,3-subunits ameliorates behavioral deficits in a mouse model of autism
- PMID: 24656250
- PMCID: PMC4079471
- DOI: 10.1016/j.neuron.2014.01.016
Enhancement of inhibitory neurotransmission by GABAA receptors having α2,3-subunits ameliorates behavioral deficits in a mouse model of autism
Abstract
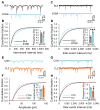
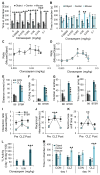
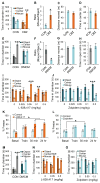
Autism spectrum disorder (ASD) may arise from increased ratio of excitatory to inhibitory neurotransmission in the brain. Many pharmacological treatments have been tested in ASD, but only limited success has been achieved. Here we report that BTBR T(+)Itpr3(tf)/J (BTBR) mice, a model of idiopathic autism, have reduced spontaneous GABAergic neurotransmission. Treatment with low nonsedating/nonanxiolytic doses of benzodiazepines, which increase inhibitory neurotransmission through positive allosteric modulation of postsynaptic GABAA receptors, improved deficits in social interaction, repetitive behavior, and spatial learning. Moreover, negative allosteric modulation of GABAA receptors impaired social behavior in C57BL/6J and 129SvJ wild-type mice, suggesting that reduced inhibitory neurotransmission may contribute to social and cognitive deficits. The dramatic behavioral improvement after low-dose benzodiazepine treatment was subunit specific-the α2,3-subunit-selective positive allosteric modulator L-838,417 was effective, but the α1-subunit-selective drug zolpidem exacerbated social deficits. Impaired GABAergic neurotransmission may contribute to ASD, and α2,3-subunit-selective positive GABAA receptor modulation may be an effective treatment.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures

References
-
- Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des. 2002;8:5–21. - PubMed
-
- Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, Mu Y, Nguyen DV, Gonzalez-Heydrich J, Wang PP, et al. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci Transl Med. 2012;4:152ra127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

