Adaptive gain control during human perceptual choice
- PMID: 24656259
- PMCID: PMC4411568
- DOI: 10.1016/j.neuron.2014.01.020
Adaptive gain control during human perceptual choice
Abstract
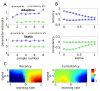
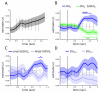
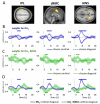
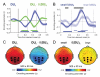
Neural systems adapt to background levels of stimulation. Adaptive gain control has been extensively studied in sensory systems but overlooked in decision-theoretic models. Here, we describe evidence for adaptive gain control during the serial integration of decision-relevant information. Human observers judged the average information provided by a rapid stream of visual events (samples). The impact that each sample wielded over choices depended on its consistency with the previous sample, with more consistent or expected samples wielding the greatest influence over choice. This bias was also visible in the encoding of decision information in pupillometric signals and in cortical responses measured with functional neuroimaging. These data can be accounted for with a serial sampling model in which the gain of information processing adapts rapidly to reflect the average of the available evidence.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Summary statistics. Weighting for the end.Atten Percept Psychophys. 2014 Jul;76(5):1253. doi: 10.3758/s13414-014-0726-z. Atten Percept Psychophys. 2014. PMID: 24961646 No abstract available.
References
-
- Aston-Jones G, Cohen JD. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol. 2005;493:99–110. - PubMed
-
- Barlow H. Sensory Communication. MIT Press; 1961. Possible principles underlying the transformation of sensory messages.
-
- Bartlett NR. Dark and light adaptation. In: Graham CH, editor. Vision and visual perception. John Wiley and Sons, Inc; New York: 1965. 1965. (New York: John Wiley and Sons, Inc.)
-
- Bogacz R, Brown E, Moehlis J, Holmes P, Cohen JD. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychol Rev. 2006;113:700–765. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

