SPOP promotes tumorigenesis by acting as a key regulatory hub in kidney cancer
- PMID: 24656772
- PMCID: PMC4443692
- DOI: 10.1016/j.ccr.2014.02.007
SPOP promotes tumorigenesis by acting as a key regulatory hub in kidney cancer
Abstract
Hypoxic stress and hypoxia-inducible factors (HIFs) play important roles in a wide range of tumors. We demonstrate that SPOP, which encodes an E3 ubiquitin ligase component, is a direct transcriptional target of HIFs in clear cell renal cell carcinoma (ccRCC). Furthermore, hypoxia results in cytoplasmic accumulation of SPOP, which is sufficient to induce tumorigenesis. This tumorigenic activity occurs through the ubiquitination and degradation of multiple regulators of cellular proliferation and apoptosis, including the tumor suppressor PTEN, ERK phosphatases, the proapoptotic molecule Daxx, and the Hedgehog pathway transcription factor Gli2. Knockdown of SPOP specifically kills ccRCC cells, indicating that it may be a promising therapeutic target. Collectively, our results indicate that SPOP serves as a regulatory hub to promote ccRCC tumorigenesis.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


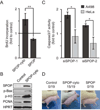
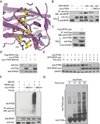
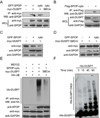
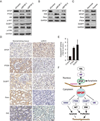
References
-
- Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol. 2008;10:1324–1332. - PubMed
-
- Brenner W, Farber G, Herget T, Lehr HA, Hengstler JG, Thuroff JW. Loss of tumor suppressor protein PTEN during renal carcinogenesis. Int J Cancer. 2002;99:53–57. - PubMed
-
- Brugarolas J. Renal-Cell Carcinoma — Molecular Pathways and Therapies. New England Journal of Medicine. 2007;356:185–187. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

