The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment
- PMID: 24656820
- PMCID: PMC4049224
- DOI: 10.1016/j.celrep.2014.02.033
The number of fetal nephron progenitor cells limits ureteric branching and adult nephron endowment
Abstract
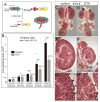
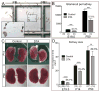
Nephrons, the functional units of the kidney, develop from progenitor cells (cap mesenchyme [CM]) surrounding the epithelial ureteric bud (UB) tips. Reciprocal signaling between UB and CM induces nephrogenesis and UB branching. Although low nephron number is implicated in hypertension and renal disease, the mechanisms that determine nephron number are obscure. To test the importance of nephron progenitor cell number, we genetically ablated 40% of these cells, asking whether this would limit kidney size and nephron number or whether compensatory mechanisms would allow the developing organ to recover. The reduction in CM cell number decreased the rate of branching, which in turn allowed the number of CM cells per UB tip to normalize, revealing a self-correction mechanism. However, the retarded UB branching impaired kidney growth, leaving a permanent nephron deficit. Thus, the number of fetal nephron progenitor cells is an important determinant of nephron endowment, largely via its effect on UB branching.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol. 2006;299:466–477. - PubMed
-
- Benz K, Campean V, Cordasic N, Karpe B, Neuhuber W, Mall G, Hartner A, Hilgers KF, Amann K. Early glomerular alterations in genetically determined low nephron number. Am J Physiol Renal Physiol. 2011;300:F521–530. - PubMed
-
- Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–1533. - PubMed
-
- Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol. 2008;313:234–245. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

