Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells
- PMID: 24656965
- PMCID: PMC5528624
- DOI: 10.1016/j.molonc.2014.02.008
Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells
Abstract
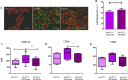
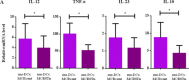
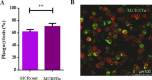
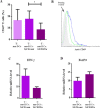
Despite the wide acceptance that glycans are centrally implicated in immunity, exactly how they contribute to the tilt immune response remains poorly defined. In this study, we sought to evaluate the impact of the malignant phenotype-associated glycan, sialyl-Tn (STn) in the function of the key orchestrators of the immune response, the dendritic cells (DCs). In high grade bladder cancer tissue, the STn antigen is significantly overexpressed and correlated with the increased expression of ST6GALNAC1 sialyltransferase. Bladder cancer tissue presenting elevated expression of ST6GALNAC1 showed a correlation with increased expression of CD1a, a marker for bladder immature DCs and showed concomitant low levels of Th1-inducing cytokines IL-12 and TNF-α. In vitro, human DCs co-incubated with STn(+) bladder cancer cells, had an immature phenotype (MHC-II(low), CD80(low) and CD86(low)) and were unresponsive to further maturation stimuli. When contacting with STn(+) cancer cells, DCs expressed significantly less IL-12 and TNF-α. Consistent with a tolerogenic DC profile, T cells that were primed by DCs pulsed with antigens derived from STn(+) cancer cells were not activated and showed a FoxP3(high) IFN-γ(low) phenotype. Blockade of STn antigens and of STn(+) glycoprotein, CD44 and MUC1, in STn(+) cancer cells was able to lower the induction of tolerance and DCs become more mature. Overall, our data suggest that STn-expressing cancer cells impair DC maturation and endow DCs with a tolerogenic function, limiting their capacity to trigger protective anti-tumour T cell responses. STn antigens and, in particular, STn(+) glycoproteins are potential targets for circumventing tumour-induced tolerogenic mechanisms.
Keywords: CD44; Dendritic cells; Immunological potency; Mucins; Sialyl-Tn; T cells.
Copyright © 2014 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Response of high-risk of recurrence/progression bladder tumours expressing sialyl-Tn and sialyl-6-T to BCG immunotherapy.Br J Cancer. 2013 Oct 15;109(8):2106-14. doi: 10.1038/bjc.2013.571. Epub 2013 Sep 24. Br J Cancer. 2013. PMID: 24064971 Free PMC article.
-
Overexpression of tumour-associated carbohydrate antigen sialyl-Tn in advanced bladder tumours.Mol Oncol. 2013 Jun;7(3):719-31. doi: 10.1016/j.molonc.2013.03.001. Epub 2013 Mar 21. Mol Oncol. 2013. PMID: 23567325 Free PMC article.
-
Delivery of alloantigens via apoptotic cells generates dendritic cells with an immature tolerogenic phenotype.Transplant Proc. 2011 Jul-Aug;43(6):2325-33. doi: 10.1016/j.transproceed.2011.06.007. Transplant Proc. 2011. PMID: 21839264
-
Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses.Front Immunol. 2018 Mar 19;9:455. doi: 10.3389/fimmu.2018.00455. eCollection 2018. Front Immunol. 2018. PMID: 29616018 Free PMC article. Review.
-
The Role of Sialyl-Tn in Cancer.Int J Mol Sci. 2016 Feb 24;17(3):275. doi: 10.3390/ijms17030275. Int J Mol Sci. 2016. PMID: 26927062 Free PMC article. Review.
Cited by
-
Mucin-Type O-Glycans: Barrier, Microbiota, and Immune Anchors in Inflammatory Bowel Disease.J Inflamm Res. 2021 Nov 13;14:5939-5953. doi: 10.2147/JIR.S327609. eCollection 2021. J Inflamm Res. 2021. PMID: 34803391 Free PMC article. Review.
-
ST6GALNAC1 promotes the invasion and migration of breast cancer cells via the EMT pathway.Genes Genomics. 2023 Nov;45(11):1367-1376. doi: 10.1007/s13258-023-01445-y. Epub 2023 Sep 25. Genes Genomics. 2023. PMID: 37747641
-
Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities.Cancers (Basel). 2018 Jun 18;10(6):207. doi: 10.3390/cancers10060207. Cancers (Basel). 2018. PMID: 29912148 Free PMC article. Review.
-
Over forty years of bladder cancer glycobiology: Where do glycans stand facing precision oncology?Oncotarget. 2017 Jul 21;8(53):91734-91764. doi: 10.18632/oncotarget.19433. eCollection 2017 Oct 31. Oncotarget. 2017. PMID: 29207682 Free PMC article. Review.
-
In-Depth Analysis of the Impact of Different Serum-Free Media on the Production of Clinical Grade Dendritic Cells for Cancer Immunotherapy.Front Immunol. 2021 Feb 5;11:593363. doi: 10.3389/fimmu.2020.593363. eCollection 2020. Front Immunol. 2021. PMID: 33613517 Free PMC article.
References
-
- Adis International, L. , 2003. Cancer vaccine THERATOPE-Biomira. Drugs R&D. 4, 236–240. - PubMed
-
- Almand, B. , Clark, J.I. , Nikitina, E. , van Beynen, J. , English, N.R. , Knight, S.C. , Carbone, D.P. , Gabrilovich, D.I. , 2001. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J. Immunol.. 166, 678–689. - PubMed
-
- Banchereau, J. , Steinman, R.M. , 1998. Dendritic cells and the control of immunity. Nature. 392, 245–252. - PubMed
-
- Beatty, J.D. , Islam, S. , North, M.E. , Knight, S.C. , Ogden, C.W. , 2004. Urine dendritic cells: a noninvasive probe for immune activity in bladder cancer?. BJU Int.. 94, 1377–1383. - PubMed
-
- Cao, Y. , Stosiek, P. , Springer, G.F. , Karsten, U. , 1996. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem. Cell Biol.. 106, 197–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

