BRD7, a tumor suppressor, interacts with p85α and regulates PI3K activity
- PMID: 24657164
- PMCID: PMC4004185
- DOI: 10.1016/j.molcel.2014.02.016
BRD7, a tumor suppressor, interacts with p85α and regulates PI3K activity
Abstract
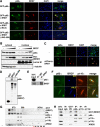
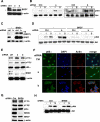
Phosphoinositide 3-kinase (PI3K) activity is important for regulating cell growth, survival, and motility. We report here the identification of bromodomain-containing protein 7 (BRD7) as a p85α-interacting protein that negatively regulates PI3K signaling. BRD7 binds to the inter-SH2 (iSH2) domain of p85 through an evolutionarily conserved region located at the C terminus of BRD7. Via this interaction, BRD7 facilitates nuclear translocation of p85α. The BRD7-dependent depletion of p85 from the cytosol impairs formation of p85/p110 complexes in the cytosol, leading to a decrease in p110 proteins and in PI3K pathway signaling. In contrast, silencing of endogenous BRD7 expression by RNAi increases the steady-state level of p110 proteins and enhances Akt phosphorylation after stimulation. These data suggest that BRD7 and p110 compete for the interaction to p85. The unbound p110 protein is unstable, leading to the attenuation of PI3K activity, which suggests how BRD7 could function as a tumor suppressor.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Bavelloni A, Santi S, Sirri A, Riccio M, Faenza I, Zini N, Cecchi S, Ferri A, Auron P, Maraldi NM, et al. Phosphatidylinositol 3-kinase translocation to the nucleus is induced by interleukin 1 and prevented by mutation of interleukin 1 receptor in human osteosarcoma Saos-2 cells. Journal of cell science. 1999;112(Pt 5):631–640. - PubMed
-
- Burrows AE, Smogorzewska A, Elledge SJ. Polybromo-associated BRG1- associated factor components BRD7 and BAF180 are critical regulators of p53 required for induction of replicative senescence. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14280–14285. - PMC - PubMed
-
- Carpenter CL, Duckworth BC, Auger KR, Cohen B, Schaffhausen BS, Cantley LC. Purification and characterization of phosphoinositide 3-kinase from rat liver. The Journal of biological chemistry. 1990;265:19704–19711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

