The phosphorylation of endogenous Nedd4-2 In Na(+)-absorbing human airway epithelial cells
- PMID: 24657276
- PMCID: PMC4022840
- DOI: 10.1016/j.ejphar.2014.03.005
The phosphorylation of endogenous Nedd4-2 In Na(+)-absorbing human airway epithelial cells
Abstract
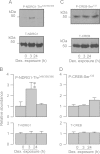
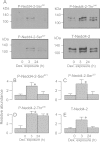
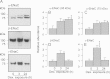
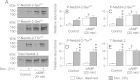
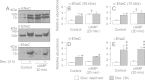
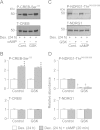
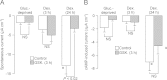
Neural precursor cell expressed, developmentally down-regulated protein 4-2 (Nedd4-2) mediates the internalisation / degradation of epithelial Na(+) channel subunits (α-, β- and γ-ENaC). Serum / glucocorticoid inducible kinase 1 (SGK1) and protein kinase A (PKA) both appear to inhibit this process by phosphorylating Nedd4-2-Ser(221), -Ser(327) and -Thr(246). This Nedd4-2 inactivation process is thought to be central to the hormonal control of Na(+) absorption. The present study of H441 human airway epithelial cells therefore explores the effects of SGK1 and / or PKA upon the phosphorylation / abundance of endogenous Nedd4-2; the surface expression of ENaC subunits, and electrogenic Na(+) transport. Effects on Nedd4-2 phosphorylation/abundance and the surface expression of ENaC were monitored by western analysis, whilst Na(+) absorption was quantified electrometrically. Acutely (20min) activating PKA in glucocorticoid-deprived (24h) cells increased the abundance of Ser(221)-phosphorylated, Ser(327)-phosphorylated and total Nedd4-2 without altering the abundance of Thr(246)-phosphorylated Nedd4-2. Activating PKA under these conditions did not cause a co-ordinated increase in the surface abundance of α-, β- and γ-ENaC and had only a very small effect upon electrogenic Na(+) absorption. Activating PKA (20min) in glucocorticoid-treated (0.2µM dexamethasone, 24h) cells, on the other hand, increased the abundance of Ser(221)-, Ser(327)- and Thr(246)-phosphorylated and total Nedd4-2; increased the surface abundance of α-, β- and γ-ENaC and evoked a clear stimulation of Na(+) transport. Chronic glucocorticoid stimulation therefore appears to allow cAMP-dependent control of Na(+) absorption by facilitating the effects of PKA upon the Nedd4-2 and ENaC subunits.
Keywords: Cellular signalling; Epithelial Na(+) channel; Protein kinase A; Pulmonary Na(+) absorption; Serum and glucocorticoid regulated kinase 1.
Copyright © 2014 The Authors. Published by Elsevier B.V. All rights reserved.
Figures




Similar articles
-
cAMP and serum and glucocorticoid-inducible kinase (SGK) regulate the epithelial Na(+) channel through convergent phosphorylation of Nedd4-2.J Biol Chem. 2004 Oct 29;279(44):45753-8. doi: 10.1074/jbc.M407858200. Epub 2004 Aug 24. J Biol Chem. 2004. PMID: 15328345
-
AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4-2.J Biol Chem. 2006 Sep 8;281(36):26159-69. doi: 10.1074/jbc.M606045200. Epub 2006 Jul 14. J Biol Chem. 2006. PMID: 16844684
-
Epithelial Na⁺ channel activity in human airway epithelial cells: the role of serum and glucocorticoid-inducible kinase 1.Br J Pharmacol. 2012 Jun;166(4):1272-89. doi: 10.1111/j.1476-5381.2012.01860.x. Br J Pharmacol. 2012. PMID: 22250980 Free PMC article.
-
Regulation of epithelial Na+ channels by aldosterone: role of Sgk1.Clin Exp Pharmacol Physiol. 2008 Feb;35(2):235-41. doi: 10.1111/j.1440-1681.2007.04844.x. Clin Exp Pharmacol Physiol. 2008. PMID: 18197893 Review.
-
New insights into the role of serum- and glucocorticoid-inducible kinase SGK1 in the regulation of renal function and blood pressure.Curr Opin Nephrol Hypertens. 2005 Jan;14(1):59-66. doi: 10.1097/00041552-200501000-00010. Curr Opin Nephrol Hypertens. 2005. PMID: 15586017 Review.
Cited by
-
Acetylation, Phosphorylation, Ubiquitination (Oh My!): Following Post-Translational Modifications on the Ubiquitin Road.Biomolecules. 2022 Mar 18;12(3):467. doi: 10.3390/biom12030467. Biomolecules. 2022. PMID: 35327659 Free PMC article. Review.
-
Two Pools of Epoxyeicosatrienoic Acids in Humans: Alterations in Salt-Sensitive Normotensive Subjects.Hypertension. 2018 Feb;71(2):346-355. doi: 10.1161/HYPERTENSIONAHA.117.10392. Epub 2017 Dec 26. Hypertension. 2018. PMID: 29279315 Free PMC article.
-
MAGED2 Enhances Expression and Function of NCC at the Cell Surface via cAMP Signaling Under Hypoxia.Cells. 2025 Jan 23;14(3):175. doi: 10.3390/cells14030175. Cells. 2025. PMID: 39936967 Free PMC article.
-
Loss of Serum Glucocorticoid-Inducible Kinase 1 SGK1 Worsens Malabsorption and Diarrhea in Microvillus Inclusion Disease (MVID).J Clin Med. 2022 Jul 19;11(14):4179. doi: 10.3390/jcm11144179. J Clin Med. 2022. PMID: 35887942 Free PMC article.
-
Serum and Glucocorticoid Regulated Kinase 1 in Sodium Homeostasis.Int J Mol Sci. 2016 Aug 10;17(8):1307. doi: 10.3390/ijms17081307. Int J Mol Sci. 2016. PMID: 27517916 Free PMC article. Review.
References
-
- Althaus M., Pichl A., Clauss W.G., Seeger W., Fronius M., Morty R.E. Nitric oxide inhibits highly selective sodium channels and the Na+/K+-ATPase in H441 cells. Am. J. Respir. Cell Mol. Biol. 2010;44:53–65. - PubMed
-
- Baines D.L. Kinases as targets for ENaC regulation. Curr. Mol. Pharmacol. CMP-EPUB. 2013:20130305–20130307. - PubMed
-
- Barker P.M., Markiewicz M., Parker K.A., Walters D.V., Strang L.B. Synergistic action of triiodothyronine and hydrocortisone on epinephrine-induced reabsorption of fetal lung liquid. Pediatr. Res. 1990;27:588–591. - PubMed
-
- Barker P.M., Olver R.E. Lung edema clearance: 20 years of progress—invited review: clearance of lung liquid during the perinatal period. J. Appl. Physiol. 2002;93:1542–1548. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

