Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity
- PMID: 24657625
- PMCID: PMC4104305
- DOI: 10.1053/j.gastro.2014.03.014
Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity
Abstract
Background & aims: The NACHT, LRR, and pyrin domain-containing protein 3 (NLRP3) inflammasome induces inflammation in response to organ injury, but little is known about its regulation. Toll-like receptors (TLRs) provide the first signal required for activation of the inflammasome and stimulate aerobic glycolysis to generate lactate. We examined whether lactate and the lactate receptor, Gi-protein-coupled receptor 81 (GPR81), regulate TLR induction of signal 1 and limit inflammasome activation and organ injury.
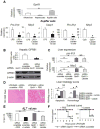
Methods: Primary mouse macrophages and human monocytes were incubated with TLR4 agonists and lactate and assayed for levels of pro-interleukin (IL)1β, NLRP3, and caspase-1 (CASP1); release of IL1β; and activation of nuclear factor-κB (NF-κB) and caspase-1. Small interfering RNAs were used to reduce levels of GPR81 and arrestin β-2 (ARRB2), and an NF-κB luciferase reporter transgene was transfected in RAW 264.7 cells. Cell lysates were analyzed by immunoprecipitation with an antibody against GPR81. Acute hepatitis was induced in C56BL/6N mice by administration of lipopolysaccharide and D-galactosamine. Acute pancreatitis was induced by administration of lipopolysaccharide and cerulein. Some mice were given intraperitoneal injections of sodium lactate or small interfering RNA against Gpr81. Activation of NF-κB in tissue macrophages was assessed in mice that expressed a reporter transgene.
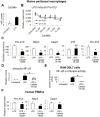
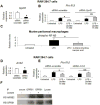
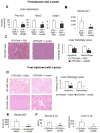
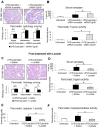
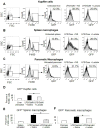
Results: In macrophages and monocytes, increasing concentrations of lactate reduced TLR4-mediated induction of Il1B, Nlrp3, and Casp1; activation of NF-κB; release of IL1β; and cleavage of CASP1. GPR81 and ARRB2 physically interacted and were required for these effects. The administration of lactate reduced inflammation and organ injury in mice with immune hepatitis; this reduction required Gpr81 dependence in vivo. Lactate also prevented activation of NF-κB in macrophages of mice, and, when given after injury, reduced the severity of acute pancreatitis and acute liver injury.
Conclusions: Lactate negatively regulates TLR induction of the NLRP3 inflammasome and production of IL1β, via ARRB2 and GPR81. Lactate could be a promising immunomodulatory therapy for patients with acute organ injury.
Keywords: Immune Regulation; Innate Immune Response; Mouse Model; Pancreas.
Copyright © 2014 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures

Comment in
-
The anti-inflammasome effect of lactate and the lactate GPR81-receptor in pancreatic and liver inflammation.Gastroenterology. 2014 Jun;146(7):1602-5. doi: 10.1053/j.gastro.2014.04.025. Epub 2014 Apr 26. Gastroenterology. 2014. PMID: 24780214 No abstract available.
Similar articles
-
Activation of N-methyl-d-aspartate receptor downregulates inflammasome activity and liver inflammation via a β-arrestin-2 pathway.Am J Physiol Gastrointest Liver Physiol. 2014 Oct 1;307(7):G732-40. doi: 10.1152/ajpgi.00073.2014. Epub 2014 Aug 7. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 25104498 Free PMC article.
-
The anti-inflammasome effect of lactate and the lactate GPR81-receptor in pancreatic and liver inflammation.Gastroenterology. 2014 Jun;146(7):1602-5. doi: 10.1053/j.gastro.2014.04.025. Epub 2014 Apr 26. Gastroenterology. 2014. PMID: 24780214 No abstract available.
-
Nuclear Receptor Subfamily 1 Group D Member 1 Regulates Circadian Activity of NLRP3 Inflammasome to Reduce the Severity of Fulminant Hepatitis in Mice.Gastroenterology. 2018 Apr;154(5):1449-1464.e20. doi: 10.1053/j.gastro.2017.12.019. Epub 2017 Dec 24. Gastroenterology. 2018. PMID: 29277561 Free PMC article.
-
NLRP3 Inflammasome-Mediated Inflammation in Acute Pancreatitis.Int J Mol Sci. 2020 Jul 29;21(15):5386. doi: 10.3390/ijms21155386. Int J Mol Sci. 2020. PMID: 32751171 Free PMC article. Review.
-
Anti-inflammatory Action of Statins in Cardiovascular Disease: the Role of Inflammasome and Toll-Like Receptor Pathways.Clin Rev Allergy Immunol. 2021 Apr;60(2):175-199. doi: 10.1007/s12016-020-08791-9. Clin Rev Allergy Immunol. 2021. PMID: 32378144 Free PMC article. Review.
Cited by
-
Lactate metabolism in human health and disease.Signal Transduct Target Ther. 2022 Sep 1;7(1):305. doi: 10.1038/s41392-022-01151-3. Signal Transduct Target Ther. 2022. PMID: 36050306 Free PMC article. Review.
-
Identification of Lactate as a Cardiac Protectant by Inhibiting Inflammation and Cardiac Hypertrophy Using a Zebrafish Acute Heart Failure Model.Pharmaceuticals (Basel). 2021 Mar 15;14(3):261. doi: 10.3390/ph14030261. Pharmaceuticals (Basel). 2021. PMID: 33803943 Free PMC article.
-
Metabolite Transporters as Regulators of Immunity.Metabolites. 2020 Oct 19;10(10):418. doi: 10.3390/metabo10100418. Metabolites. 2020. PMID: 33086598 Free PMC article. Review.
-
Bifidobacterium mechanisms of immune modulation and tolerance.Gut Microbes. 2023 Dec;15(2):2291164. doi: 10.1080/19490976.2023.2291164. Epub 2023 Dec 6. Gut Microbes. 2023. PMID: 38055306 Free PMC article. Review.
-
Glycolysis modulates efferocytosis in a noncanonical manner.Nat Metab. 2023 Mar;5(3):360-361. doi: 10.1038/s42255-023-00746-6. Nat Metab. 2023. PMID: 36797419 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

