Sinorhizobium meliloti chemoreceptor McpU mediates chemotaxis toward host plant exudates through direct proline sensing
- PMID: 24657863
- PMCID: PMC4018860
- DOI: 10.1128/AEM.00115-14
Sinorhizobium meliloti chemoreceptor McpU mediates chemotaxis toward host plant exudates through direct proline sensing
Abstract
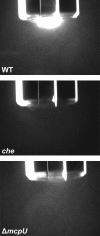
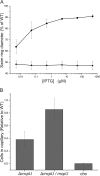
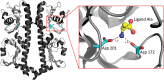
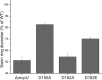
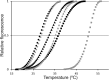
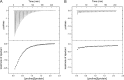
Bacterial chemotaxis is an important attribute that aids in establishing symbiosis between rhizobia and their legume hosts. Plant roots and seeds exude a spectrum of molecules into the soil to attract their bacterial symbionts. The alfalfa symbiont Sinorhizobium meliloti possesses eight chemoreceptors to sense its environment and mediate chemotaxis toward its host. The methyl accepting chemotaxis protein McpU is one of the more abundant S. meliloti chemoreceptors and an important sensor for the potent attractant proline. We established a dominant role of McpU in sensing molecules exuded by alfalfa seeds. Mass spectrometry analysis determined that a single germinating seed exudes 3.72 nmol of proline, producing a millimolar concentration near the seed surface which can be detected by the chemosensory system of S. meliloti. Complementation analysis of the mcpU deletion strain verified McpU as the key proline sensor. A structure-based homology search identified tandem Cache (calcium channels and chemotaxis receptors) domains in the periplasmic region of McpU. Conserved residues Asp-155 and Asp-182 of the N-terminal Cache domain were determined to be important for proline sensing by evaluating mutant strains in capillary and swim plate assays. Differential scanning fluorimetry revealed interaction of the isolated periplasmic region of McpU (McpU40-284) with proline and the importance of Asp-182 in this interaction. Using isothermal titration calorimetry, we determined that proline binds with a Kd (dissociation constant) of 104 μM to McpU40-284, while binding was abolished when Asp-182 was substituted by Glu. Our results show that McpU is mediating chemotaxis toward host plants by direct proline sensing.
Figures


References
-
- Uren NC. 2007. Types, amounts, and possible functions of compounds released into the rhizosphere by soil-grown plants, p 19–40 In Pinton R, Varanini Z, Nannipiero P. (ed), The rhizosphere: biochemistry and organic substances at the soil-plant interface. CRC Press, New York, NY
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

