Bioretrosynthetic construction of a didanosine biosynthetic pathway
- PMID: 24657930
- PMCID: PMC4017637
- DOI: 10.1038/nchembio.1494
Bioretrosynthetic construction of a didanosine biosynthetic pathway
Abstract
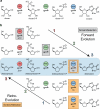
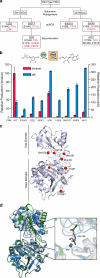
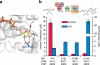
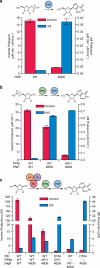
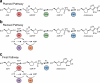
Concatenation of engineered biocatalysts into multistep pathways markedly increases their utility, but the development of generalizable assembly methods remains a major challenge. Herein we evaluate 'bioretrosynthesis', which is an application of the retrograde evolution hypothesis, for biosynthetic pathway construction. To test bioretrosynthesis, we engineered a pathway for synthesis of the antiretroviral nucleoside analog didanosine (2',3'-dideoxyinosine). Applying both directed evolution- and structure-based approaches, we began pathway construction with a retro-extension from an engineered purine nucleoside phosphorylase and evolved 1,5-phosphopentomutase to accept the substrate 2,3-dideoxyribose 5-phosphate with a 700-fold change in substrate selectivity and threefold increased turnover in cell lysate. A subsequent retrograde pathway extension, via ribokinase engineering, resulted in a didanosine pathway with a 9,500-fold change in nucleoside production selectivity and 50-fold increase in didanosine production. Unexpectedly, the result of this bioretrosynthetic step was not a retro-extension from phosphopentomutase but rather the discovery of a fortuitous pathway-shortening bypass via the engineered ribokinase.
Figures
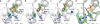
Similar articles
-
Design and directed evolution of a dideoxy purine nucleoside phosphorylase.Protein Eng Des Sel. 2010 Aug;23(8):607-16. doi: 10.1093/protein/gzq033. Epub 2010 Jun 4. Protein Eng Des Sel. 2010. PMID: 20525731 Free PMC article.
-
Nucleoside phosphorylases from clostridium perfringens in the synthesis of 2',3'-dideoxyinosine.Nucleosides Nucleotides Nucleic Acids. 2010 Jun;29(4-6):445-8. doi: 10.1080/15257771003741422. Nucleosides Nucleotides Nucleic Acids. 2010. PMID: 20544534
-
Engineering biosynthetic enzymes for industrial natural product synthesis.Nat Prod Rep. 2020 Aug 19;37(8):1122-1143. doi: 10.1039/c9np00071b. Nat Prod Rep. 2020. PMID: 32364202 Review.
-
Absorption and intestinal metabolism of purine dideoxynucleosides and an adenosine deaminase-activated prodrug of 2',3'-dideoxyinosine in the mesenteric vein cannulated rat ileum.J Pharm Sci. 1998 May;87(5):569-77. doi: 10.1021/js9703582. J Pharm Sci. 1998. PMID: 9572907
-
Engineering the third wave of biocatalysis.Nature. 2012 May 9;485(7397):185-94. doi: 10.1038/nature11117. Nature. 2012. PMID: 22575958 Review.
Cited by
-
Industrially Relevant Enzyme Cascades for Drug Synthesis and Their Ecological Assessment.Int J Mol Sci. 2022 Mar 25;23(7):3605. doi: 10.3390/ijms23073605. Int J Mol Sci. 2022. PMID: 35408960 Free PMC article. Review.
-
Repurposing Modular Polyketide Synthases and Non-ribosomal Peptide Synthetases for Novel Chemical Biosynthesis.Front Mol Biosci. 2020 May 15;7:87. doi: 10.3389/fmolb.2020.00087. eCollection 2020. Front Mol Biosci. 2020. PMID: 32500080 Free PMC article. Review.
-
Biosynthesis of Arabinoside from Sucrose and Nucleobase via a Novel Multi-Enzymatic Cascade.Biomolecules. 2024 Sep 3;14(9):1107. doi: 10.3390/biom14091107. Biomolecules. 2024. PMID: 39334873 Free PMC article.
-
An engineered aldolase enables the biocatalytic synthesis of 2'-functionalized nucleoside analogues.Nat Synth. 2025;4(2):156-166. doi: 10.1038/s44160-024-00671-w. Epub 2024 Nov 5. Nat Synth. 2025. PMID: 39959249 Free PMC article.
-
Recent Developments and Applications of Biocatalytic and Chemoenzymatic Synthesis for the Generation of Diverse Classes of Drugs.Curr Pharm Biotechnol. 2024;25(4):448-467. doi: 10.2174/0113892010238984231019085154. Curr Pharm Biotechnol. 2024. PMID: 37885105
References
-
- Savile CK, et al. Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture. Science. 2010;329:305–309. - PubMed
-
- Liang J, et al. Development of a biocatalytic process as an alternative to the (−)-DIP-Cl-mediated asymmetric reduction of a key intermediate of montelukast. Org. Process. Res. Dev. 2010;14:193–198.
-
- Bornscheuer UT, et al. Engineering the third wave of biocatalysis. Nature. 2012;485:185–194. - PubMed
-
- Paddon CJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
- R01 GM061606/GM/NIGMS NIH HHS/United States
- T32 NS007491/NS/NINDS NIH HHS/United States
- T32NS07491/NS/NINDS NIH HHS/United States
- R25 GM062459/GM/NIGMS NIH HHS/United States
- R01 GM077189/GM/NIGMS NIH HHS/United States
- T32 GM065086/GM/NIGMS NIH HHS/United States
- 5T32GM008320/GM/NIGMS NIH HHS/United States
- S10 RR026915/RR/NCRR NIH HHS/United States
- T90 DA022873/DA/NIDA NIH HHS/United States
- T32 GM008320/GM/NIGMS NIH HHS/United States
- GM077189/GM/NIGMS NIH HHS/United States
- GM079419/GM/NIGMS NIH HHS/United States
- R01 GM079419/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

