Nuclear transport of galectin-3 and its therapeutic implications
- PMID: 24657939
- PMCID: PMC4108496
- DOI: 10.1016/j.semcancer.2014.03.004
Nuclear transport of galectin-3 and its therapeutic implications
Abstract
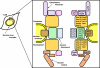
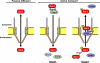
Galectin-3, a member of β-galactoside-binding gene family is a multi-functional protein, which regulates pleiotropic biological functions such as cell growth, cell adhesion, cell-cell interactions, apoptosis, angiogenesis and mRNA processing. Its unique structure enables it to interact with a plethora of ligands in a carbohydrate dependent or independent manner. Galectin-3 is mainly a cytosolic protein, but can easily traverse the intracellular and plasma membranes to translocate into the nucleus, mitochondria or get externalized. Depending on the cell type, specific experimental conditions in vitro, cancer type and stage, galectin-3 has been reported to be exclusively cytoplasmic, predominantly nuclear or distributed between the two compartments. In this review we have summarized the dynamics of galectin-3 shuttling between the nucleus and the cytoplasm, the nuclear transport mechanisms of galectin-3, how its specific interactions with the members of β-catenin signaling pathways affect tumor progression, and its implications as a therapeutic target.
Keywords: Beta catenin; Galectin-3; Nuclear cytoplasmic transport.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures

References
-
- Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269(33):20807–10. - PubMed
-
- Hirabayashi J, Hashidate T, Arata Y, Nishi N, Nakamura T, Hirashima M, et al. Oligosaccharide specificity of galectins: a search by frontal affinity chromatography. Biochimica et biophysica acta. 2002;1572(2-3):232–54. - PubMed
-
- Davidson PJ, Li SY, Lohse AG, Vandergaast R, Verde E, Pearson A, et al. Transport of galectin-3 between the nucleus and cytoplasm. I. Conditions and signals for nuclear import. Glycobiology. 2006;16(7):602–11. - PubMed
-
- Li SY, Davidson PJ, Lin NY, Patterson RJ, Wang JL, Arnoys EJ. Transport of galectin-3 between the nucleus and cytoplasm. II. Identification of the signal for nuclear export. Glycobiology. 2006;16(7):612–22. - PubMed
-
- Davidson PJ, Davis MJ, Patterson RJ, Ripoche MA, Poirier F, Wang JL. Shuttling of galectin-3 between the nucleus and cytoplasm. Glycobiology. 2002;12(5):329–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

