Ultrasound-assisted siRNA delivery via arginine-grafted bioreducible polymer and microbubbles targeting VEGF for ovarian cancer treatment
- PMID: 24657947
- PMCID: PMC4025968
- DOI: 10.1016/j.jconrel.2014.03.025
Ultrasound-assisted siRNA delivery via arginine-grafted bioreducible polymer and microbubbles targeting VEGF for ovarian cancer treatment
Abstract
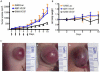
The major drawback hampering siRNA therapies from being more widely accepted in clinical practice is its insufficient accumulation at the target site mainly due to poor cellular uptake and rapid degradation in serum. Therefore, we designed a novel polymeric siRNA carrier system, which would withstand serum-containing environments and tested its performance in vitro as well as in vivo. Delivering siRNA with a system combining an arginine-grafted bioreducible polymer (ABP), microbubbles (MBs), and ultrasound technology (US) we were able to synergize the advantages each delivery system owns individually, and created our innovative siRNA-ABP-MB (SAM) complexes. SAM complexes show significantly higher siRNA uptake and VEGF protein knockdown in vitro with serum-containing media when compared to naked siRNA, and 25k-branched-polyethylenimine (bPEI) representing the current standard in nonviral gene therapy. SAM complexes activated by US are also able to improve siRNA uptake in tumor tissue resulting in decelerating tumor growth in vivo.
Keywords: Cancer; Microbubbles; RNAi; Ultrasound; VEGF; siRNA.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures

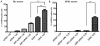


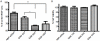
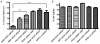

References
-
- Hammond SM, Caudy AA, Hannon GJ. Post-transcriptional gene silencing by double-stranded RNA. Nat. Rev. Genet. 2001;2:110–119. - PubMed
-
- Semenza GL. Targeting HIF-1 for cancer therapy. Nature Reviews Cancer. 2003;3:721–732. - PubMed
-
- Ferrara N. VEGF-A: a critical regulator of blood vessel growth. European cytokine network. 2009;20:158–163. - PubMed
-
- Wannenes F, Ciafré SA, Niola F, Frajese G, Farace MG. Vector-based RNA interference against vascular endothelial growth factor-A significantly limits vascularization and growth of prostate cancer in vivo. Cancer Gene Therapy. 2005;12:926–934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

