Intrastriatal transplantation of adenovirus-generated induced pluripotent stem cells for treating neuropathological and functional deficits in a rodent model of Huntington's disease
- PMID: 24657963
- PMCID: PMC4006485
- DOI: 10.5966/sctm.2013-0151
Intrastriatal transplantation of adenovirus-generated induced pluripotent stem cells for treating neuropathological and functional deficits in a rodent model of Huntington's disease
Abstract
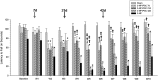
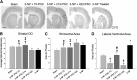
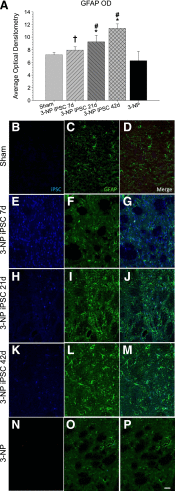
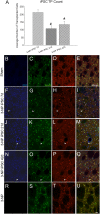
Induced pluripotent stem cells (iPSCs) show considerable promise for cell replacement therapies for Huntington's disease (HD). Our laboratory has demonstrated that tail-tip fibroblasts, reprogrammed into iPSCs via two adenoviruses, can survive and differentiate into neuronal lineages following transplantation into healthy adult rats. However, the ability of these cells to survive, differentiate, and restore function in a damaged brain is unknown. To this end, adult rats received a regimen of 3-nitropropionic acid (3-NP) to induce behavioral and neuropathological deficits that resemble HD. At 7, 21, and 42 days after the initiation of 3-NP or vehicle, the rats received intrastriatal bilateral transplantation of iPSCs. All rats that received 3-NP and vehicle treatment displayed significant motor impairment, whereas those that received iPSC transplantation after 3-NP treatment had preserved motor function. Histological analysis of the brains of these rats revealed significant decreases in optical densitometric measures in the striatum, lateral ventricle enlargement, as well as an increase in striosome size in all rats receiving 3-NP when compared with sham rats. The 3-NP-treated rats given transplants of iPSCs in the 7- or 21-day groups did not exhibit these deficits. Transplantation of iPSCs at the late-stage (42-day) time point did not protect against the 3-NP-induced neuropathology, despite preserving motor function. Transplanted iPSCs were found to survive and differentiate into region-specific neurons in the striatum of 3-NP rats, at all transplantation time points. Taken together, these results suggest that transplantation of adenovirus-generated iPSCs may provide a potential avenue for therapeutic treatment of HD.
Keywords: 3-Nitropropionic acid; Adenovirus; Huntington’s disease; Stem cell; Transplantation; iPSC.
Figures


References
-
- The Huntington’s Disease Collaborative Research Group A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. - PubMed
-
- Estrada Sánchez AM, Mejía-Toiber J, Massieu L. Excitotoxic neuronal death and the pathogenesis of Huntington’s disease. Arch Med Res. 2008;39:265–276. - PubMed
-
- Bachoud-Lévi A-C. Neural grafts in Huntington’s disease: Viability after 10 years. Lancet Neurol. 2009;8:979–981. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

