An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia
- PMID: 24658077
- PMCID: PMC7730561
- DOI: 10.1038/nm.3468
An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia
Abstract
The pathophysiology of ineffective erythropoiesis in β-thalassemia is poorly understood. We report that RAP-011, an activin receptor IIA (ActRIIA) ligand trap, improved ineffective erythropoiesis, corrected anemia and limited iron overload in a mouse model of β-thalassemia intermedia. Expression of growth differentiation factor 11 (GDF11), an ActRIIA ligand, was increased in splenic erythroblasts from thalassemic mice and in erythroblasts and sera from subjects with β-thalassemia. Inactivation of GDF11 decreased oxidative stress and the amount of α-globin membrane precipitates, resulting in increased terminal erythroid differentiation. Abnormal GDF11 expression was dependent on reactive oxygen species, suggesting the existence of an autocrine amplification loop in β-thalassemia. GDF11 inactivation also corrected the abnormal ratio of immature/mature erythroblasts by inducing apoptosis of immature erythroblasts through the Fas-Fas ligand pathway. Taken together, these observations suggest that ActRIIA ligand traps may have therapeutic relevance in β-thalassemia by suppressing the deleterious effects of GDF11, a cytokine which blocks terminal erythroid maturation through an autocrine amplification loop involving oxidative stress and α-globin precipitation.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details are available in the online version of the paper.
Figures
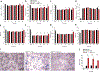



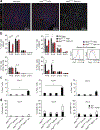

References
-
- Higgs DR, Engel JD & Stamatoyannopoulos G Thalassaemia. Lancet 379, 373–383 (2012). - PubMed
-
- Kihm AJ et al. An abundant erythroid protein that stabilizes free α-haemoglobin. Nature 417, 758–763 (2002). - PubMed
-
- Ribeil JA et al. Ineffective erythropoiesis in β-thalassemia. ScientificWorldJournal 2013, 1–11 (2013).
-
- Sorensen S, Rubin E, Polster H, Mohandas N & Schrier S The role of membrane skeletal-associated α-globin in the pathophysiology of β-thalassemia. Blood 75, 1333–1336 (1990). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

