Moyamoya disease-associated protein mysterin/RNF213 is a novel AAA+ ATPase, which dynamically changes its oligomeric state
- PMID: 24658080
- PMCID: PMC3963067
- DOI: 10.1038/srep04442
Moyamoya disease-associated protein mysterin/RNF213 is a novel AAA+ ATPase, which dynamically changes its oligomeric state
Abstract
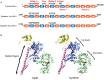
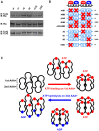
Moyamoya disease is an idiopathic human cerebrovascular disorder that is characterized by progressive stenosis and abnormal collateral vessels. We recently identified mysterin/RNF213 as its first susceptibility gene, which encodes a 591-kDa protein containing enzymatically active P-loop ATPase and ubiquitin ligase domains and is involved in proper vascular development in zebrafish. Here we demonstrate that mysterin further contains two tandem AAA+ ATPase modules and forms huge ring-shaped oligomeric complex. AAA+ ATPases are known to generally mediate various biophysical and mechanical processes with the characteristic ring-shaped structure. Fluorescence correlation spectroscopy and biochemical evaluation suggested that mysterin dynamically changes its oligomeric forms through ATP/ADP binding and hydrolysis cycles. Thus, the moyamoya disease-associated gene product is a unique protein that functions as ubiquitin ligase and AAA+ ATPase, which possibly contributes to vascular development through mechanical processes in the cell.
Figures



References
-
- Takeuchi K. & Shimizu K. Hypoplasia of the bilateral internal carotid arteries. Brain Nerve 9, 37–43 (1957).
-
- Kuroda S. & Houkin K. Moyamoya disease: current concepts and future perspectives. Lancet Neurol 7, 1056–1066, http://dx.doi.org/10.1016/S1474-4422(08)70240-0 (2008). - DOI - PubMed
-
- Scott R. M. & Smith E. R. Moyamoya disease and moyamoya syndrome. N. Engl. J. Med. 360, 1226–1237, http://dx.doi.org/10.1056/NEJMra0804622 (2009). - DOI - PubMed
-
- Suzuki J. & Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch. Neurol. 20, 288–299 (1969). - PubMed
-
- Fukui M. Guidelines for the diagnosis and treatment of spontaneous occlusion of the circle of Willis (‘moyamoya’ disease). Research Committee on Spontaneous Occlusion of the Circle of Willis (Moyamoya Disease) of the Ministry of Health and Welfare, Japan. Clin. Neurol. Neurosurg. 99 Suppl 2, S238–240 (1997). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

