EphA4 activation of c-Abl mediates synaptic loss and LTP blockade caused by amyloid-β oligomers
- PMID: 24658113
- PMCID: PMC3962387
- DOI: 10.1371/journal.pone.0092309
EphA4 activation of c-Abl mediates synaptic loss and LTP blockade caused by amyloid-β oligomers
Abstract
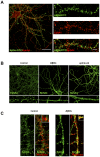
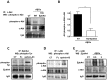
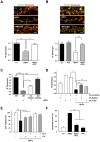
The early stages of Alzheimer's disease are characterised by impaired synaptic plasticity and synapse loss. Here, we show that amyloid-β oligomers (AβOs) activate the c-Abl kinase in dendritic spines of cultured hippocampal neurons and that c-Abl kinase activity is required for AβOs-induced synaptic loss. We also show that the EphA4 receptor tyrosine kinase is upstream of c-Abl activation by AβOs. EphA4 tyrosine phosphorylation (activation) is increased in cultured neurons and synaptoneurosomes exposed to AβOs, and in Alzheimer-transgenic mice brain. We do not detect c-Abl activation in EphA4-knockout neurons exposed to AβOs. More interestingly, we demonstrate EphA4/c-Abl activation is a key-signalling event that mediates the synaptic damage induced by AβOs. According to this results, the EphA4 antagonistic peptide KYL and c-Abl inhibitor STI prevented i) dendritic spine reduction, ii) the blocking of LTP induction and iii) neuronal apoptosis caused by AβOs. Moreover, EphA4-/- neurons or sh-EphA4-transfected neurons showed reduced synaptotoxicity by AβOs. Our results are consistent with EphA4 being a novel receptor that mediates synaptic damage induced by AβOs. EphA4/c-Abl signalling could be a relevant pathway involved in the early cognitive decline observed in Alzheimer's disease patients.
Conflict of interest statement
Figures



References
-
- Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, et al. (2004) Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron 44: 181–193. - PubMed
-
- Klein WL (2013) Synaptotoxic, Amyloid-β Oligomers: A Molecular Basis for the Cause, Diagnosis, and Treatment of Alzheimer's Disease? J Alzheimers Dis 33 Suppl 1S49–S65. - PubMed
-
- Estrada LD, Zanlungo SM, Alvarez AR (2011) c-Abl tyrosine kinase signaling: a new player in AD tau pathology. Curr Alzheimer Res 6: 643–651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

