Granulocyte/macrophage colony-stimulating factor influences angiogenesis by regulating the coordinated expression of VEGF and the Ang/Tie system
- PMID: 24658178
- PMCID: PMC3962430
- DOI: 10.1371/journal.pone.0092691
Granulocyte/macrophage colony-stimulating factor influences angiogenesis by regulating the coordinated expression of VEGF and the Ang/Tie system
Abstract
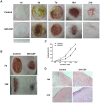
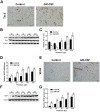
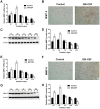
Granulocyte/macrophage colony-stimulating factor (GM-CSF) can accelerate wound healing by promoting angiogenesis. The biological effects of GM-CSF in angiogenesis and the corresponding underlying molecular mechanisms, including in the early stages of primitive endothelial tubule formation and the later stages of new vessel maturation, have only been partially clarified. This study aimed to investigate the effects of GM-CSF on angiogenesis and its regulatory mechanisms. Employing a self-controlled model (Sprague-Dawley rats with deep partial-thickness burn wounds), we determined that GM-CSF can increase VEGF expression and decrease the expression ratio of Ang-1/Ang-2 and the phosphorylation of Tie-2 in the early stages of the wound healing process, which promotes the degradation of the basement membrane and the proliferation of endothelial cells. At later stages of wound healing, GM-CSF can increase the expression ratio of Ang-1/Ang-2 and the phosphorylation of Tie-2 and maintain a high VEGF expression level. Consequently, pericyte coverages were higher, and the basement membrane became more integrated in new blood vessels, which enhanced the barrier function of blood vessels. In summary, we report here that increased angiogenesis is associated with GM-CSF treatment, and we indicate that VEGF and the Ang/Tie system may act as angiogenic mediators of the healing effect of GM-CSF on burn wounds.
Conflict of interest statement
Figures




Similar articles
-
GM-CSF ameliorates microvascular barrier integrity via pericyte-derived Ang-1 in wound healing.Wound Repair Regen. 2017 Nov;25(6):933-943. doi: 10.1111/wrr.12608. Epub 2018 Feb 16. Wound Repair Regen. 2017. PMID: 29328541
-
Granulocyte/macrophage colony-stimulating factor treatment of human chronic ulcers promotes angiogenesis associated with de novo vascular endothelial growth factor transcription in the ulcer bed.Br J Dermatol. 2006 Jan;154(1):34-41. doi: 10.1111/j.1365-2133.2005.06925.x. Br J Dermatol. 2006. PMID: 16403091 Clinical Trial.
-
Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis.Lab Invest. 2006 Nov;86(11):1172-84. doi: 10.1038/labinvest.3700476. Epub 2006 Sep 11. Lab Invest. 2006. PMID: 16969369
-
Practical Application of Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) in Patients with Wounds.Surg Technol Int. 2018 Jun 1;32:61-66. Surg Technol Int. 2018. PMID: 29611156 Review.
-
Ang-1 and VEGF: central regulators of angiogenesis.Mol Cell Biochem. 2025 Feb;480(2):621-637. doi: 10.1007/s11010-024-05010-3. Epub 2024 Apr 23. Mol Cell Biochem. 2025. PMID: 38652215 Review.
Cited by
-
Single Dose of N-Acetylcysteine in Local Anesthesia Increases Expression of HIF1α, MAPK1, TGFβ1 and Growth Factors in Rat Wound Healing.Int J Mol Sci. 2021 Aug 12;22(16):8659. doi: 10.3390/ijms22168659. Int J Mol Sci. 2021. PMID: 34445365 Free PMC article.
-
An artificial neural network classification method employing longitudinally monitored immune biomarkers to predict the clinical outcome of critically ill COVID-19 patients.PeerJ. 2022 Dec 12;10:e14487. doi: 10.7717/peerj.14487. eCollection 2022. PeerJ. 2022. PMID: 36530391 Free PMC article.
-
New Insight into Molecular and Hormonal Connection in Andrology.Int J Mol Sci. 2021 Nov 2;22(21):11908. doi: 10.3390/ijms222111908. Int J Mol Sci. 2021. PMID: 34769341 Free PMC article. Review.
-
Gametocytes of the Malaria Parasite Plasmodium falciparum Interact With and Stimulate Bone Marrow Mesenchymal Cells to Secrete Angiogenetic Factors.Front Cell Infect Microbiol. 2018 Mar 1;8:50. doi: 10.3389/fcimb.2018.00050. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29546035 Free PMC article.
-
Allogeneic transplantation of programmable cells of monocytic origin (PCMO) improves angiogenesis and tissue recovery in critical limb ischemia (CLI): a translational approach.Stem Cell Res Ther. 2018 Apr 27;9(1):117. doi: 10.1186/s13287-018-0871-8. Stem Cell Res Ther. 2018. PMID: 29703251 Free PMC article.
References
-
- Martin P (1997) Wound healing–aiming for perfect skin regeneration. Science 276: 75–81. - PubMed
-
- Augustin HG, Koh GY, Thurston G, Alitalo K (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 10: 165–177. - PubMed
-
- Thurston G, Suri C, Smith K (1999) Leakage-resistant blood vessels in mice transgenically over expressing angiopoitin-1. Science. pp. 2511–2514. - PubMed
-
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, et al. (2000) Vascular specific growth factors and blood vessel formation. Nature 407: 242–248. - PubMed
-
- Mann A, Breuhahn K, Schirmacher P (2001) Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing.Stimulation of keratinocyte proliferation,granulation tissue formation and vascularization. J Invest Dermatol 117: 1382–1390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

