P21 activated kinases: structure, regulation, and functions
- PMID: 24658305
- PMCID: PMC4160339
- DOI: 10.4161/sgtp.28003
P21 activated kinases: structure, regulation, and functions
Abstract
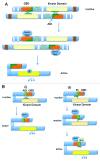
The p21 activated kinases (Paks) are well known effector proteins for the Rho GTPases Cdc42 and Rac. The Paks contain 6 members, which fall into 2 families of proteins. The first family consists of Paks 1, 2, and 3, and the second consists of Paks 4, 5, and 6. While some of the Paks are ubiquitously expressed, others have more restrictive tissue specificity. All of them are found in the nervous system. Studies using cell culture, transgenic mice, and knockout mice, have revealed important roles for the Paks in cytoskeletal organization and in many aspects of cell growth and development. This review discusses the basic structures of the Paks, and their roles in cell growth, development, and in cancer.
Keywords: neurobiology; oncogenesis; p21-activated kinases; pak; protein kinases; substrates.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
