Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation
- PMID: 24658423
- PMCID: PMC3962409
- DOI: 10.1371/journal.pone.0092427
Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation
Abstract
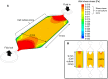
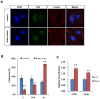
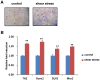
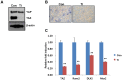
Shear stress activates cellular signaling involved in cellular proliferation, differentiation, and migration. However, the mechanisms of mesenchymal stem cell (MSC) differentiation under interstitial flow are not fully understood. Here, we show the increased osteogenic differentiation of MSCs under exposure to constant, extremely low shear stress created by osmotic pressure-induced flow in a microfluidic chip. The interstitial level of shear stress in the proposed microfluidic system stimulated nuclear localization of TAZ (transcriptional coactivator with PDZ-binding motif), a transcriptional modulator of MSCs, activated TAZ target genes such as CTGF and Cyr61, and induced osteogenic differentiation. TAZ-depleted cells showed defects in shear stress-induced osteogenic differentiation. In shear stress induced cellular signaling, Rho signaling pathway was important forthe nuclear localization of TAZ. Taken together, these results suggest that TAZ is an important mediator of interstitial flow-driven shear stress signaling in osteoblast differentiation of MSCs.
Conflict of interest statement
Figures

Similar articles
-
Icariin stimulates osteogenic differentiation of rat bone marrow stromal stem cells by increasing TAZ expression.Biomed Pharmacother. 2017 Jul;91:581-589. doi: 10.1016/j.biopha.2017.04.019. Epub 2017 May 6. Biomed Pharmacother. 2017. PMID: 28486190
-
Type I collagen deposition via osteoinduction ameliorates YAP/TAZ activity in 3D floating culture clumps of mesenchymal stem cell/extracellular matrix complexes.Stem Cell Res Ther. 2018 Dec 7;9(1):342. doi: 10.1186/s13287-018-1085-9. Stem Cell Res Ther. 2018. PMID: 30526677 Free PMC article.
-
IGF1 promotes osteogenic differentiation of mesenchymal stem cells derived from rat bone marrow by increasing TAZ expression.Biochem Biophys Res Commun. 2013 Apr 5;433(2):226-31. doi: 10.1016/j.bbrc.2013.02.088. Epub 2013 Mar 5. Biochem Biophys Res Commun. 2013. PMID: 23473758
-
[Regulation of differentiation of mesenchymal stem cells by the Hippo pathway effectors TAZ/YAP].Yi Chuan. 2013 Nov;35(11):1283-90. doi: 10.3724/sp.j.1005.2013.01283. Yi Chuan. 2013. PMID: 24579311 Review. Chinese.
-
Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress.Biomech Model Mechanobiol. 2010 Dec;9(6):659-70. doi: 10.1007/s10237-010-0206-x. Epub 2010 Mar 23. Biomech Model Mechanobiol. 2010. PMID: 20309603 Review.
Cited by
-
Study on the Expansion Dynamics of MDCK Epithelium by Interstitial Flow Using a Traction Force-Measurable Microfluidic Chip.Materials (Basel). 2021 Feb 16;14(4):935. doi: 10.3390/ma14040935. Materials (Basel). 2021. PMID: 33669345 Free PMC article.
-
Bone physiological microenvironment and healing mechanism: Basis for future bone-tissue engineering scaffolds.Bioact Mater. 2021 Apr 22;6(11):4110-4140. doi: 10.1016/j.bioactmat.2021.03.043. eCollection 2021 Nov. Bioact Mater. 2021. PMID: 33997497 Free PMC article. Review.
-
Recent Advances in Mechanically Loaded Human Mesenchymal Stem Cells for Bone Tissue Engineering.Int J Mol Sci. 2020 Aug 13;21(16):5816. doi: 10.3390/ijms21165816. Int J Mol Sci. 2020. PMID: 32823645 Free PMC article. Review.
-
Biomechanical, biophysical and biochemical modulators of cytoskeletal remodelling and emergent stem cell lineage commitment.Commun Biol. 2023 Jan 19;6(1):75. doi: 10.1038/s42003-022-04320-w. Commun Biol. 2023. PMID: 36658332 Free PMC article. Review.
-
Role of the Hippo Pathway in Fibrosis and Cancer.Cells. 2019 May 16;8(5):468. doi: 10.3390/cells8050468. Cells. 2019. PMID: 31100975 Free PMC article. Review.
References
-
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, et al. (2011) Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183. - PubMed
-
- Halder G, Dupont S, Piccolo S (2012) Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nature reviews Molecular cell biology 13: 591–600. - PubMed
-
- Hong JH, Hwang ES, McManus MT, Amsterdam A, Tian Y, et al. (2005) TAZ, a transcriptional modulator of mesenchymal stem cell differentiation. Science 309: 1074–1078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

