Exogenous asymmetric dimethylarginine (ADMA) in pathogenesis of ischemia-reperfusion-induced gastric lesions: interaction with protective nitric oxide (NO) and calcitonin gene-related peptide (CGRP)
- PMID: 24658439
- PMCID: PMC3975433
- DOI: 10.3390/ijms15034946
Exogenous asymmetric dimethylarginine (ADMA) in pathogenesis of ischemia-reperfusion-induced gastric lesions: interaction with protective nitric oxide (NO) and calcitonin gene-related peptide (CGRP)
Abstract
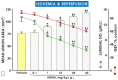
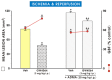
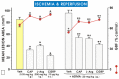
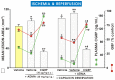
Asymmetric dimethylarginine (ADMA) is an endogenous nitric oxide (NO) synthesis inhibitor and pro-inflammatory factor. We investigated the role of ADMA in rat gastric mucosa compromised through 30 min of gastric ischemia (I) and 3 h of reperfusion (R). These I/R animals were pretreated with ADMA with or without the combination of L-arginine, calcitonin gene-related peptide (CGRP) or a small dose of capsaicin, all of which are known to afford protection against gastric lesions, or with a farnesoid X receptor (FXR) agonist, GW 4064, to increase the metabolism of ADMA. In the second series, ADMA was administered to capsaicin-denervated rats. The area of gastric damage was measured with planimetry, gastric blood flow (GBF) was determined by H2-gas clearance, and plasma ADMA and CGRP levels were determined using ELISA and RIA. ADMA significantly increased I/R-induced gastric injury while significantly decreasing GBF, the luminal NO content, and the plasma level of CGRP. This effect of ADMA was significantly attenuated by pretreatment with CGRP, L-arginine, capsaicin, or a PGE2 analogue. In GW4064 pretreated animals, the I/R injury was significantly reduced and this effect was abolished by co-treatment with ADMA. I/R damage potentiated by ADMA was exacerbated in capsaicin-denervated animals with a further reduction of CGRP. Plasma levels of IL-10 were significantly decreased while malonylodialdehyde (MDA) and plasma TNF-α contents were significantly increased by ADMA. In conclusion, ADMA aggravates I/R-induced gastric lesions due to a decrease of GBF, which is mediated by a fall in NO and CGRP release, and the enhancement of lipid peroxidation and its pro-inflammatory properties.
Figures

Similar articles
-
Ghrelin-induced gastroprotection against ischemia-reperfusion injury involves an activation of sensory afferent nerves and hyperemia mediated by nitric oxide.Eur J Pharmacol. 2006 Apr 24;536(1-2):171-81. doi: 10.1016/j.ejphar.2006.02.032. Epub 2006 Feb 28. Eur J Pharmacol. 2006. PMID: 16581065
-
Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, interacts with gastric oxidative metabolism and enhances stress-induced gastric lesions.J Physiol Pharmacol. 2012 Oct;63(5):515-24. J Physiol Pharmacol. 2012. PMID: 23211305
-
Role of sensory afferent nerves, lipid peroxidation and antioxidative enzymes in the carbon monoxide-induced gastroprotection against stress ulcerogenesis.J Physiol Pharmacol. 2016 Oct;67(5):717-729. J Physiol Pharmacol. 2016. PMID: 28011952
-
The impact of asymmetric dimethylarginine (ADAMA), the endogenous nitric oxide (NO) synthase inhibitor, to the pathogenesis of gastric mucosal damage.Curr Pharm Des. 2013;19(1):90-7. doi: 10.2174/13816128130113. Curr Pharm Des. 2013. PMID: 22950506 Review.
-
Asymmetric dimethylarginine: a novel biomarker of gastric mucosal injury?World J Gastroenterol. 2011 May 7;17(17):2178-80. doi: 10.3748/wjg.v17.i17.2178. World J Gastroenterol. 2011. PMID: 21633526 Free PMC article. Review.
Cited by
-
Hydrogen Sulfide and Carbon Monoxide Protect Gastric Mucosa Compromised by Mild Stress Against Alendronate Injury.Dig Dis Sci. 2016 Nov;61(11):3176-3189. doi: 10.1007/s10620-016-4280-5. Epub 2016 Aug 19. Dig Dis Sci. 2016. PMID: 27541924 Free PMC article.
-
Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing.Molecules. 2015 May 19;20(5):9099-123. doi: 10.3390/molecules20059099. Molecules. 2015. PMID: 25996214 Free PMC article. Review.
-
Carbon Monoxide and Nitric Oxide as Examples of the Youngest Class of Transmitters.Int J Mol Sci. 2021 Jun 2;22(11):6029. doi: 10.3390/ijms22116029. Int J Mol Sci. 2021. PMID: 34199647 Free PMC article. Review.
-
Neuropathic pain releasing calcitonin gene related peptide protects against stroke in rats.Am J Transl Res. 2020 Jan 15;12(1):54-69. eCollection 2020. Am J Transl Res. 2020. PMID: 32051737 Free PMC article.
-
Carbon monoxide released from its pharmacological donor, tricarbonyldichlororuthenium (II) dimer, accelerates the healing of pre-existing gastric ulcers.Br J Pharmacol. 2017 Oct;174(20):3654-3668. doi: 10.1111/bph.13968. Epub 2017 Aug 30. Br J Pharmacol. 2017. PMID: 28768046 Free PMC article.
References
-
- Leiper J., Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542–548. - PubMed
-
- Leiper J., Vallance P. The synthesis and metabolism of asymmetric dimethylarginine (ADMA) Eur. J. Clin. Pharmacol. 2006;62:33–38.
-
- Beltowski J., Kedra A. Asymmetric dimethylarginine (ADMA) as a target for pharmacotherapy. Pharmacol. Rep. 2006;58:159–178. - PubMed
-
- Wilcken D.E., Sim A.S., Wang J., Wang X.L. Asymmetric dimethylarginine (ADMA) in vascular renal and hepatic disease and the regulatory role of l-arginine on its metabolism. Mol. Genet. Metab. 2007;91:309–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

