Bovine induced pluripotent stem cells are more resistant to apoptosis than testicular cells in response to mono-(2-ethylhexyl) phthalate
- PMID: 24658443
- PMCID: PMC3975437
- DOI: 10.3390/ijms15035011
Bovine induced pluripotent stem cells are more resistant to apoptosis than testicular cells in response to mono-(2-ethylhexyl) phthalate
Abstract
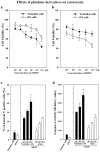
Although the androgen receptor (AR) has been implicated in the promotion of apoptosis in testicular cells (TSCs), the molecular pathway underlying AR-mediated apoptosis and its sensitivity to environmental hormones in TSCs and induced pluripotent stem cells (iPSCs) remain unclear. We generated the iPSCs from bovine TSCs via the electroporation of OCT4. The established iPSCs were supplemented with leukemia inhibitory factor and bone morphogenetic protein 4 to maintain and stabilize the expression of stemness genes and their pluripotency. Apoptosis signaling was assessed after exposure to mono-(2-ethylhexyl) phthalate (MEHP), the active metabolite of di-(2-ethylhexyl) phthalate. Here, we report that iPSCs were more resistant to MEHP-induced apoptosis than were original TSCs. MEHP also repressed the expression of AR and inactivated WNT signaling, and then led to the commitment of cells to apoptosis via the cyclin dependent kinase inhibitor p21CIP1. The loss of the frizzed receptor 7 and the gain of p21CIP were responsible for the stimulatory effect of MEHP on AR-mediated apoptosis. Our results suggest that testicular iPSCs can be used to study the signaling pathways involved in the response to environmental disruptors, and to assess the toxicity of environmental endocrine disruptors in terms of the maintenance of stemness and pluripotency.
Figures








References
-
- Lyche J.L., Gutleb A.C., Bergman A., Eriksen G.S., Murk A.J., Ropstad E., Saunders M., Skaare J.U. Reproductive and developmental toxicity of phthalates. J. Toxicol. Environ. Health Part B. 2009;12:225–249. - PubMed
-
- Jurewicz J., Hanke W. Exposure to phthalates: Reproductive outcome and children health A review of epidemiological studies. Int. J. Occup. Med. Environ. Health. 2011;24:115–141. - PubMed
-
- Awal M.A., Kurohmaru M., Ishii M., Andriana B.B., Kanai Y., Hayashi Y. Mono-(2-ethylhexyl) phthalate (MEHP) induces spermatogenic cell apoptosis in guinea pig testes at prepubertal stage in vitro. Int. J. Toxicol. 2004;23:349–355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

