Largazole pharmacokinetics in rats by LC-MS/MS
- PMID: 24658499
- PMCID: PMC3967229
- DOI: 10.3390/md12031623
Largazole pharmacokinetics in rats by LC-MS/MS
Abstract
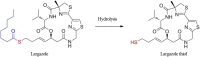
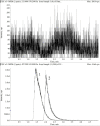
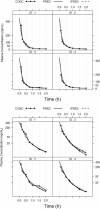
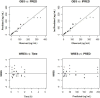
A highly sensitive and specific LC-MS/MS method for the quantitation of largazole thiol, the active species of the marine-derived preclinical histone deacetylase inhibitor, largazole (prodrug), was developed and validated. Largazole thiol was extracted with ethyl acetate from human or rat plasma along with the internal standard, harmine. Samples were separated on an Onyx Monolithic C18 column by a stepwise gradient elution with 0.1% formic acid in methanol and 0.1% aqueous formic acid employing multiple reaction monitoring (MRM) detection. Linear calibration curves were obtained in the range of 12.5-400 ng/mL with 200 µL of human plasma. The overall intra-day precision was from 3.87% to 12.6%, and the inter-day precision was from 7.12% to 9.8%. The accuracy at low, medium and high concentrations ranged from 101.55% to 105.84%. Plasma protein bindings of largazole thiol in human and rat plasma as determined by an ultrafiltration method were 90.13% and 77.14%, respectively. Plasma drug concentrations were measured by this LC-MS/MS method. The pharmacokinetics of largazole thiol in rats was studied following i.v. administration at 10 mg/kg and found to follow a two-compartment model. Largazole thiol was rapidly eliminated from systemic circulation within 2 h. The established LC-MS/MS method is suitable for the analysis of largazole thiol in human plasma, as well.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

