miR-17/20 sensitization of breast cancer cells to chemotherapy-induced apoptosis requires Akt1
- PMID: 24658544
- PMCID: PMC4011585
- DOI: 10.18632/oncotarget.1804
miR-17/20 sensitization of breast cancer cells to chemotherapy-induced apoptosis requires Akt1
Abstract
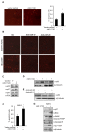
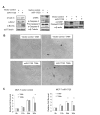
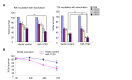
The serine threonine kinase Akt1 has been implicated in the control of cellular metabolism, survival and growth. Herein, disruption of the ubiquitously expressed member of the Akt family of genes, Akt1, in the mouse, demonstrates a requirement for Akt1 in miRNA-mediated cellular apoptosis. The miR-17/20 cluster is known to inhibit breast cancer cellular proliferation through G1/S cell cycle arrest via binding to the cyclin D1 3'UTR. Here we show that miR-17/20 overexpression sensitizes cells to apoptosis induced by either Doxorubicin or UV irradiation in MCF-7 cells via Akt1. miR-17/20 mediates apoptosis via increased p53 expression which promotes Akt degradation. Akt1⁻/⁻ mammary epithelial cells which express Akt2 and Akt3 demonstrated increased apoptosis to DNA damaging agents. Akt1 deficiency abolished the miR-17/20-mediated apoptosis. These results demonstrated a novel pathway through which miR17/20 regulate p53 and Akt controlling breast cancer cell apoptosis.
Figures


Similar articles
-
Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice.Cancer Res. 2007 Jan 1;67(1):167-77. doi: 10.1158/0008-5472.CAN-06-3782. Cancer Res. 2007. PMID: 17210696
-
Loss of Akt1 or Akt2 delays mammary tumor onset and suppresses tumor growth rate in MTB-IGFIR transgenic mice.BMC Cancer. 2013 Aug 7;13:375. doi: 10.1186/1471-2407-13-375. BMC Cancer. 2013. PMID: 23919516 Free PMC article.
-
miR-222 induces Adriamycin resistance in breast cancer through PTEN/Akt/p27kip1 pathway.Tumour Biol. 2016 Nov;37(11):15315-15324. doi: 10.1007/s13277-016-5341-2. Epub 2016 Oct 4. Tumour Biol. 2016. PMID: 27699665
-
Distinct biological roles for the akt family in mammary tumor progression.Cancer Res. 2010 Jun 1;70(11):4260-4. doi: 10.1158/0008-5472.CAN-10-0266. Epub 2010 Apr 27. Cancer Res. 2010. PMID: 20424120 Free PMC article. Review.
-
Distinct functions of AKT isoforms in breast cancer: a comprehensive review.Cell Commun Signal. 2019 Nov 21;17(1):154. doi: 10.1186/s12964-019-0450-3. Cell Commun Signal. 2019. PMID: 31752925 Free PMC article. Review.
Cited by
-
miR-30b suppresses the progression of breast cancer through inhibition of the PI3K/Akt signaling pathway by targeting Derlin-1.Transl Cancer Res. 2019 Feb;8(1):180-190. doi: 10.21037/tcr.2019.01.21. Transl Cancer Res. 2019. PMID: 35116747 Free PMC article.
-
ApoptomiRs of Breast Cancer: Basics to Clinics.Front Genet. 2016 Sep 29;7:175. doi: 10.3389/fgene.2016.00175. eCollection 2016. Front Genet. 2016. PMID: 27746811 Free PMC article. Review.
-
MicroRNA-423 promotes proliferation, migration and invasion and induces chemoresistance of endometrial cancer cells.Exp Ther Med. 2018 Nov;16(5):4213-4224. doi: 10.3892/etm.2018.6710. Epub 2018 Sep 7. Exp Ther Med. 2018. PMID: 30344696 Free PMC article.
-
The related miRNAs involved in doxorubicin resistance or sensitivity of various cancers: an update.Cancer Chemother Pharmacol. 2021 Nov;88(5):771-793. doi: 10.1007/s00280-021-04337-8. Epub 2021 Sep 12. Cancer Chemother Pharmacol. 2021. PMID: 34510251 Review.
-
Doxycycline inhibits breast cancer EMT and metastasis through PAR-1/NF-κB/miR-17/E-cadherin pathway.Oncotarget. 2017 Aug 24;8(62):104855-104866. doi: 10.18632/oncotarget.20418. eCollection 2017 Dec 1. Oncotarget. 2017. PMID: 29285218 Free PMC article.
References
-
- Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–5. - PubMed
-
- Majewski N, Nogueira V, Bhaskar P, et al. Hexokinase-mitochondria interaction mediated by Akt is required to inhibit apoptosis in the presence or absence of Bax and Bak. Mol Cell. 2004;16:819–30. - PubMed
-
- Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

