Overexpression of lymphoid enhancer-binding factor 1 (LEF1) in solid-pseudopapillary neoplasms of the pancreas
- PMID: 24658583
- PMCID: PMC4318228
- DOI: 10.1038/modpathol.2014.40
Overexpression of lymphoid enhancer-binding factor 1 (LEF1) in solid-pseudopapillary neoplasms of the pancreas
Abstract
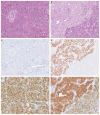
Solid-pseudopapillary neoplasms are rare, but are distinctive pancreatic tumors of low-malignant potential. While the histogenesis of these tumors is unclear, they are often associated with gain-of-function mutations in the catenin (cadherin-associated protein), beta 1 (88 kDa), or CTNNB1 gene, resulting in nuclear accumulation of CTNNB1. CTNNB1 is a central component of the Wnt signaling pathway and mediates gene expression through the lymphoid enhancer-binding factor 1 (LEF1) /T-cell factor transcription complex. Although LEF1 has a pivotal role in the transactivation of Wnt/CTNNB1 responsive genes, the status of LEF1 in solid-pseudopapillary neoplasms and other pancreatic tumors has not been examined. We analyzed both LEF1 and CTNNB1 in a large cohort of pancreatic tumors (n=155). In all cases of solid-pseudopapillary neoplasms including surgical resections (n=27) and cytologic samples (n=8) had strong and diffuse nuclear labeling for both LEF1 and CTNNB1. The surrounding uninvolved pancreatic parenchyma was devoid of any LEF1 staining. All resection and cytologic specimens from well-differentiated pancreatic neuroendocrine tumors (n=44; n=29, respectively), high-grade pancreatic neuroendocrine carcinomas (n=2; n=1), pancreatic ductal adenocarcinomas (n=25; n=12), and acinar cell carcinomas (n=9; n=2) studied were negative for both nuclear LEF1 and CTNNB1. However, nuclear LEF1 and CTNNB1 were detected in all four resected pancreatoblastomas (no cytologic specimens were available for immunolabeling), but primarily centered around and within squamoid corpuscles. In summary, abnormal CTNNB1 accumulation was accompanied by nuclear LEF1 overexpression in both solid-pseudopapillary neoplasms and pancreatoblastomas. But, in contrast to pancreatoblastomas, a diffuse, nuclear labeling was observed in solid-pseudopapillary neoplasms and further implicates the CTNNB1/LEF1 transcriptional complex in the development of solid-pseudopapillary neoplasms. In addition, as part of an immunohistochemical panel, LEF1 can be a useful ancillary stain in the diagnosis of solid-pseudopapillary neoplasms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Frantz VK. Atlas of Tumor Pathology, section VII, Fascicles 27 and 28. Armed Forces Institute of Pathology; Washington, DC: 1959. Tumors of the pancreas; pp. 32–33.
-
- Butte JM, Brennan MF, Gonen M, et al. Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg. 2011;15:350–357. - PubMed
-
- Martin RC, Klimstra DS, Brennan MF, et al. Solid-pseudopapillary tumor of the pancreas: a surgical enigma? Ann Surg Oncol. 2002;9:35–40. - PubMed
-
- Hruban RH, Pitman MB, Klimstra DS. Atlas of Tumor Pathology, 4th Series, Fascicle 6. American Registry of Pathology; Washington, DC: 2007. Solid-Pseudopapillary Neoplasms. Tumors of the pancreas; pp. 231–250.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

