LcrQ blocks the role of LcrF in regulating the Ysc-Yop type III secretion genes in Yersinia pseudotuberculosis
- PMID: 24658611
- PMCID: PMC3962397
- DOI: 10.1371/journal.pone.0092243
LcrQ blocks the role of LcrF in regulating the Ysc-Yop type III secretion genes in Yersinia pseudotuberculosis
Abstract
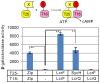
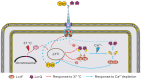
Pathogenic Yersinia species employ the Ysc-Yop type III secretion system (T3SS) encoded by a highly conserved pYV virulence plasmid to export the virulence effectors into host cells. The Ysc-Yop T3SS is tightly regulated by multiple contributing proteins that function at different levels. However, systematic transcriptional regulation analysis of Ysc-Yop T3SS is lacking and the detailed mechanism under this regulation process is still elusive. Aimed at systematically characterizing transcriptional regulations of all T3SS genes in Y. pseudotuberculosis, we amplified 97 non-coding fragments from the pYV plasmid and analyzed transcriptional responses of the T3SS genes under different growth conditions. Transcriptions of T3SS genes were induced at 37°C and genes encoding T3SS effectors were highly induced by further depletion of Ca2+. The temperature induced gene transcription process is mediated by modules encoded on the chromosome, while the Ca2+ depletion-induced process is controlled by the positive regulatory protein LcrF as well as the negative regulatory protein LcrQ. In this process, LcrQ shares the same targets with LcrF and the effect of LcrQ is dependent on the presence of LcrF. Furthermore, over-expression of LcrF showed the same phenotype as that of the lcrQ mutant strain and intracellular amount balance of LcrQ and LcrF is important in T3SS regulation. When the expression level of LcrF exceeds LcrQ, expression of the Ysc-Yop T3SS genes is activated and vice versa. Together, these data support a model in which LcrQ blocks the activation role of LcrF in regulating the transcription of T3SS genes in Yersinia.
Conflict of interest statement
Figures




Similar articles
-
RcsB positively regulates the Yersinia Ysc-Yop type III secretion system by activating expression of the master transcriptional regulator LcrF.Environ Microbiol. 2015 Apr;17(4):1219-33. doi: 10.1111/1462-2920.12556. Epub 2014 Jul 25. Environ Microbiol. 2015. PMID: 25039908
-
LcrQ Coordinates with the YopD-LcrH Complex To Repress lcrF Expression and Control Type III Secretion by Yersinia pseudotuberculosis.mBio. 2021 Jun 29;12(3):e0145721. doi: 10.1128/mBio.01457-21. Epub 2021 Jun 22. mBio. 2021. PMID: 34154409 Free PMC article.
-
IscR is essential for yersinia pseudotuberculosis type III secretion and virulence.PLoS Pathog. 2014 Jun 12;10(6):e1004194. doi: 10.1371/journal.ppat.1004194. eCollection 2014 Jun. PLoS Pathog. 2014. PMID: 24945271 Free PMC article.
-
Links between type III secretion and extracytoplasmic stress responses in Yersinia.Front Cell Infect Microbiol. 2012 Oct 10;2:125. doi: 10.3389/fcimb.2012.00125. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23087910 Free PMC article. Review.
-
Yersinia Type III Secretion System Master Regulator LcrF.J Bacteriol. 2015 Dec 7;198(4):604-14. doi: 10.1128/JB.00686-15. J Bacteriol. 2015. PMID: 26644429 Free PMC article. Review.
Cited by
-
Type III secretion by Yersinia pseudotuberculosis is reliant upon an authentic N-terminal YscX secretor domain.Mol Microbiol. 2022 Apr;117(4):886-906. doi: 10.1111/mmi.14880. Epub 2022 Feb 8. Mol Microbiol. 2022. PMID: 35043994 Free PMC article.
-
BfvR, an AraC-Family Regulator, Controls Biofilm Formation and pH6 Antigen Production in Opposite Ways in Yersinia pestis Biovar Microtus.Front Cell Infect Microbiol. 2018 Oct 2;8:347. doi: 10.3389/fcimb.2018.00347. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30333962 Free PMC article.
-
Characterization of a Minimal Type of Promoter Containing the -10 Element and a Guanine at the -14 or -13 Position in Mycobacteria.J Bacteriol. 2017 Oct 3;199(21):e00385-17. doi: 10.1128/JB.00385-17. Print 2017 Nov 1. J Bacteriol. 2017. PMID: 28784819 Free PMC article.
-
Interrelationship between type three secretion system and metabolism in pathogenic bacteria.Front Cell Infect Microbiol. 2014 Oct 27;4:150. doi: 10.3389/fcimb.2014.00150. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25386411 Free PMC article. Review.
-
The Regulatory Circuit Underlying Downregulation of a Type III Secretion System in Yersinia enterocolitica by Transcription Factor OmpR.Int J Mol Sci. 2022 Apr 26;23(9):4758. doi: 10.3390/ijms23094758. Int J Mol Sci. 2022. PMID: 35563149 Free PMC article.
References
-
- Navarro L, Alto NM, Dixon JE (2005) Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr Opin Microbiol 8: 21–27. - PubMed
-
- Viboud GI, Bliska JB (2005) Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol 59: 69–89. - PubMed
-
- Juris SJ, Shao F, Dixon JE (2002) Yersinia effectors target mammalian signalling pathways. Cell Microbiol 4: 201–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

