Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis
- PMID: 24658694
- PMCID: PMC4189821
- DOI: 10.7326/M13-1484
Accuracy of fecal immunochemical tests for colorectal cancer: systematic review and meta-analysis
Abstract
Background: Performance characteristics of fecal immunochemical tests (FITs) to screen for colorectal cancer (CRC) have been inconsistent.
Purpose: To synthesize data about the diagnostic accuracy of FITs for CRC and identify factors affecting its performance characteristics.
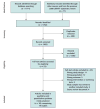
Data sources: Online databases, including MEDLINE and EMBASE, and bibliographies of included studies from 1996 to 2013.
Study selection: All studies evaluating the diagnostic accuracy of FITs for CRC in asymptomatic, average-risk adults.
Data extraction: Two reviewers independently extracted data and critiqued study quality.
Data synthesis: Nineteen eligible studies were included and meta-analyzed. The pooled sensitivity, specificity, positive likelihood ratio, and negative likelihood ratio of FITs for CRC were 0.79 (95% CI, 0.69 to 0.86), 0.94 (CI, 0.92 to 0.95), 13.10 (CI, 10.49 to 16.35), 0.23 (CI, 0.15 to 0.33), respectively, with an overall diagnostic accuracy of 95% (CI, 93% to 97%). There was substantial heterogeneity between studies in both the pooled sensitivity and specificity estimates. Stratifying by cutoff value for a positive test result or removal of discontinued FIT brands resulted in homogeneous sensitivity estimates. Sensitivity for CRC improved with lower assay cutoff values for a positive test result (for example, 0.89 [CI, 0.80 to 0.95] at a cutoff value less than 20 µg/g vs. 0.70 [CI, 0.55 to 0.81] at cutoff values of 20 to 50 µg/g) but with a corresponding decrease in specificity. A single-sample FIT had similar sensitivity and specificity as several samples, independent of FIT brand.
Limitations: Only English-language articles were included. Lack of data prevented complete subgroup analyses by FIT brand.
Conclusion: Fecal immunochemical tests are moderately sensitive, are highly specific, and have high overall diagnostic accuracy for detecting CRC. Diagnostic performance of FITs depends on the cutoff value for a positive test result.
Primary funding source: National Institute of Diabetes and Digestive and Kidney Diseases and National Cancer Institute.
Conflict of interest statement
Figures




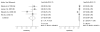
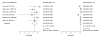

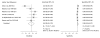
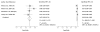
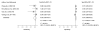
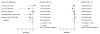

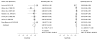

References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472–7. - PubMed
-
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467–71. - PubMed
-
- Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365–71. - PubMed
-
- Lieberman DA, Weiss DG, Veterans Affairs Cooperative Study Group 380 One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med. 2001;345:555–60. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
