Older patients with chronic myeloid leukemia (≥65 years) profit more from higher imatinib doses than younger patients: a subanalysis of the randomized CML-Study IV
- PMID: 24658964
- PMCID: PMC4050299
- DOI: 10.1007/s00277-014-2041-0
Older patients with chronic myeloid leukemia (≥65 years) profit more from higher imatinib doses than younger patients: a subanalysis of the randomized CML-Study IV
Abstract
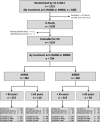
The impact of imatinib dose on response rates and survival in older patients with chronic myeloid leukemia in chronic phase has not been studied well. We analyzed data from the German CML-Study IV, a randomized five-arm treatment optimization study in newly diagnosed BCR-ABL-positive chronic myeloid leukemia in chronic phase. Patients randomized to imatinib 400 mg/day (IM400) or imatinib 800 mg/day (IM800) and stratified according to age (≥65 years vs. <65 years) were compared regarding dose, response, adverse events, rates of progression, and survival. The full 800 mg dose was given after a 6-week run-in period with imatinib 400 mg/day. The dose could then be reduced according to tolerability. A total of 828 patients were randomized to IM400 or IM800. Seven hundred eighty-four patients were evaluable (IM400, 382; IM800, 402). One hundred ten patients (29 %) on IM400 and 83 (21 %) on IM800 were ≥65 years. The median dose per day was lower for patients ≥65 years on IM800, with the highest median dose in the first year (466 mg/day for patients ≥65 years vs. 630 mg/day for patients <65 years). Older patients on IM800 achieved major molecular remission and deep molecular remission as fast as younger patients, in contrast to standard dose imatinib with which older patients achieved remissions much later than younger patients. Grades 3 and 4 adverse events were similar in both age groups. Five-year relative survival for older patients was comparable to that of younger patients. We suggest that the optimal dose for older patients is higher than 400 mg/day. ClinicalTrials.gov identifier: NCT00055874
Figures


References
-
- Rohrbacher M, Berger U, Hochhaus A, Metzgeroth G, Adam K, Lahaye T, Saussele S, Müller MC, Hasford J, Heimpel H, Hehlmann R. Clinical trials underestimate the age of chronic myeloid leukemia (CML) patients. Incidence and median age of Ph/BCR-ABL-positive CML and other chronic myeloproliferative disorders in a representative area in Germany. Leukemia. 2009;23(3):602–604. doi: 10.1038/leu.2008.245. - DOI - PubMed
-
- Björkholm M, Ohm L, Eloranta S, Derolf A, Hultcrantz M, Sjöberg J, Andersson T, Höglund M, Richter J, Landgren O, Kristinsson SY, Dickman PW. Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. J Clin Oncol. 2011;29(18):2514–2520. doi: 10.1200/JCO.2011.34.7146. - DOI - PMC - PubMed
-
- Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Cronin K, Chen HS, Feuer EJ, Stinchcomb DG, Edwards BK (eds) (based on November 9 SEER data submission, posted to the SEER web site, 2010 ) SEER cancer statistics review, 1975–2007: median age of cancer patients at diagnosis, 2003–2007 by primary cancer site, race and sex. http://seer.cancer.gov/csr/1975_2007/results_single/sect_01_table.11_2pg..., National Cancer Institute, Bethesda, MD
-
- Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson RA, or the IRIS Investigators Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

