Terbutaline pump maintenance therapy after threatened preterm labour for reducing adverse neonatal outcomes
- PMID: 24659357
- PMCID: PMC11193541
- DOI: 10.1002/14651858.CD010800.pub2
Terbutaline pump maintenance therapy after threatened preterm labour for reducing adverse neonatal outcomes
Abstract
Background: After successful inhibition of threatened preterm labour women are at high risk of recurrent preterm labour. Terbutaline pump maintenance therapy has been used to reduce adverse neonatal outcomes. This review replaces an earlier Cochrane review, published in 2002, which is no longer being updated by the team.
Objectives: To determine the effectiveness of terbutaline pump maintenance therapy after threatened preterm labour in reducing adverse neonatal outcomes.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 January 2014) and reference lists of retrieved studies.
Selection criteria: Randomised controlled trials comparing terbutaline pump therapy with alternative therapy, placebo, or no therapy after arrest of threatened preterm labour.
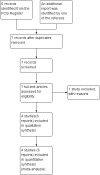
Data collection and analysis: Two review authors independently assessed the studies for inclusion and then extracted data as eligible for inclusion in qualitative and quantitative synthesis (meta-analysis).
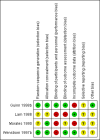
Main results: Four studies were included with a total of 234 women randomised. The overall methodological quality of the included studies was mixed; two studies provided very little information on study methods, there was high sample attrition in one study and in three studies the risk of performance bias was high. We found no strong evidence that terbutaline maintenance therapy offered any advantages over saline placebo or oral terbutaline maintenance therapy in reducing adverse neonatal outcomes by prolonging pregnancy among women with arrested preterm labour. The mean difference (MD) for gestational age at birth was -0.14 weeks (95% confidence interval (CI) -1.66 to 1.38) for terbutaline pump therapy compared with saline placebo pump for two trials combined. One trial reported a risk ratio (RR) of 1.17 (95% CI 0.79 to 1.73) for preterm birth (less than 37 completed weeks) and a RR of 0.97 (95% CI 0.51 to 1.84) of very preterm birth (less than 34 completed weeks) for terbutaline pump compared with saline placebo pump. We found no evidence that terbutaline pump therapy was associated with statistically significant reductions in infant respiratory distress syndrome, or neonatal intensive care unit admission compared with placebo. Compared with oral terbutaline, we found no evidence that pump therapy increased the rate of therapy continuation, or reduced the rate of infant complications or maternal hospital re-admissions. One study suggested that pump therapy resulted in significantly increased weekly cost/woman, $580 versus $12.50 (P < 0.01). No data were reported on long-term infant outcomes.
Authors' conclusions: We found no evidence that terbutaline pump maintenance therapy decreased adverse neonatal outcomes. Taken together with the lack of evidence of benefit, its substantial expense and the lack of information on the safety of the therapy do not support its use in the management of arrested preterm labour. Future use should only be in the context of well-conducted, adequately powered randomised controlled trials.
Conflict of interest statement
None known.
Figures












Update of
- doi: 10.1002/14651858.CD010800
References
References to studies included in this review
Guinn 1998b {published data only}
-
- Elliott J, Lam, Morrison J. Terbutaline [letter]. American Journal of Obstetrics and Gynecology 1999;180(6 Pt 1):1593‐5. - PubMed
-
- Guinn DA, Goepfert AR, Owen J, Wenstrom KD, Hauth JC. Terbutaline pump maintenance therapy for prevention of preterm delivery: a double‐blind trial. American Journal of Obstetrics and Gynecology 1998;179(4):874‐8. - PubMed
Lam 1988 {published data only}
-
- Lam F, Gill P, Smith M. Comparison of portable subcutaneous terbutaline pump and oral terbutaline treatment for long‐term tocolysis: a randomized clinical trial. Proceedings of 8th Annual Meeting of the Society of Perinatal Obstetricians; 1988 Feb 3‐6; Las Vegas, Nevada, USA. 1988:Abstract no: 400.
Morales 1990 {published data only}
-
- Morales WJ, Marko BH. Adjusted oral terbutaline vs subcutaneous terbutaline pump therapy in the management of preterm labor. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:149.
Wenstrom 1997b {published data only}
-
- Wenstrom K, Weiner C, Merrill D, Niebyl J. A placebo‐controlled trial of the terbutaline (T) pump for prevention of preterm delivery [abstract]. American Journal of Obstetrics and Gynecology 1995;172:416. - PubMed
-
- Wenstrom KD, Weiner CP, Merrill D, Niebyl J. A placebo‐controlled randomized trial of the terbutaline pump for prevention of preterm delivery. American Journal of Perinatology 1997;14(2):87‐91. - PubMed
References to studies excluded from this review
Morrison 1993 {published data only}
-
- Morrison JC. Terbutaline administered by subcutaneous programmable infusion pump vs oral terbutaline in those women with recurrent preterm labor. Personal communication 1993.
Additional references
Ananth 2006
-
- Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. Journal of Maternal Fetal Neonatal Medicine 2006;19(12):773‐82. - PubMed
Beck 2010
Callaghan 2006
-
- Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics 2006;118:1566‐73. - PubMed
Chawanpaiboon 2009
-
- Chawanpaiboon S, Sutantawibul A, Pimol K, Sirisomboon R, Worapitaksanond S. Preliminary study: comparison of the efficacy of progesterone and nifedipine in inhibiting threatened preterm labour in Siriraj Hospital. Thai Journal of Obstetrics and Gynaecology 2009;17(1):23‐9.
Chawanpaiboon 2011
-
- Chawanpaiboon S, Wanitpongpan P, Titapant V, Kanokpongsakdi S, Wantanasiri C, Pimol K, et al. Nifidipine for inhibiting threatened preterm labour in Siriraj Hospital. Siriraj Medical Journal 2008;60(3):111‐3.
Elliott 2004
-
- Elliott JP, Istwan NB, Rhea D, Stanziano G. The occurrence of adverse events in women receiving continuous subcutaneous terbutaline therapy. American Journal of Obstetrics and Gynecology 2004;191(4):1277‐82. - PubMed
Elliott 2013
FDA 2011
-
- FDA U.S. Food, Drug Administration. Terbutaline: Label Change ‐ Warnings Against Use for Treatment of Preterm Labor. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHuma... (accessed 18 October 2013) 2011, February 17.
Goldenberg 2002
-
- Goldenberg RL. High‐risk pregnancy series: an expert's view. Obstetrics & Gynecology 2002;100(5):1020‐37. - PubMed
Goldenberg 2008
Guinn 1998a
-
- Guinn DA, Goepfert AR, Owen J, Wenstrom KD, Hauth JC. Terbutaline pump maintenance therapy for prevention of preterm delivery: a double‐blind trial. American Journal of Obstetrics and Gynecology 1998;179(4):874‐8. - PubMed
Hayes 2008
-
- Hayes, Inc. Continuous subcutaneous terbutaline infusion for treatment of preterm labor. Lansdale, PA: Healthcare Technology Brief Publication, 2008.
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2009
-
- Hofmeyr GJ. Antenatal corticosteroids for women at risk of preterm birth: RHL commentary (last revised: 2 February 2009). The WHO Reproductive Health Library. http://apps.who.int/rhl/pregnancy_childbirth/complications/preterm_birth... (accessed 18 October 2013) 2009.
Iams 2009
-
- Iams JD, Romero R, Creasy RK. Preterm labour and birth. In: 566‐567 editor(s). Maternal‐Fetal Medicine. Saunders Elsevier: sixth, 2009.
Lam 1998
-
- Lam F, Elliott J, Jones JS, Katz M, Knuppel RA, Morrison J, et al. Clinical issues surrounding the use of terbutaline sulfate for preterm labor. Obstetrical and Gynecological Survey 1998;53(11 Suppl):S85‐S95. - PubMed
Pennell 2007
-
- Pennell CE, Jacobsson B, Williams SM, Buus RM, Muglia LJ, Dolan SM, et al. Genetic epidemiologic studies of preterm birth: guidelines for research. American Journal of Obstetrics & Gynecology 2007;196(2):107‐18. - PubMed
Perry 1995
-
- Perry KG Jr, Morrison JC, Rust OA, Sullivan CA, Martin RW, Naef RW 3rd. Incidence of adverse cardiopulmonary effects with low‐dose continuous terbutaline infusion. American Journal of Obstetrics and Gynecology 1995;173(4):1273‐7. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Slotkin 2003
-
- Slotkin TA, Auman JT, Seidler FJ. Ontogenesis of β‐adrenoceptor signaling: implications for perinatal physiology and for fetal effects of tocolytic drugs. Journal of Pharmacology and Experimental Therapeutics 2003;306(1):1‐7. - PubMed
Soloff 2000
-
- Soloff MS, Jeng YJ, Copland JA, Strakova Z, Hoare S. Signal pathways mediating oxytocin stimulation of prostaglandin synthesis in select target cells. Experimental Physiology 2000;85:51S. - PubMed
Valenzuela 2000
-
- Valenzuela GJ, Sanchez‐Ramos L, Romero R, Silver HM, Koltun WD, Millar L, et al. Maintenance treatment of preterm labor with the oxytocin antagonist atosiban. The Atosiban PTL‐098 Study Group. American Journal of Obstetrics and Gynecology 2000;182(5):1184. - PubMed
Vatish 2005
-
- Vatish M, Grom K, Bennett P, Thornton S. Preterm labour: managing risk in clinical practice. In: Norman J, Greer I editor(s). Management of Preterm Labour. Cambridge: Cambridge University Press, 2005:192‐208.
Wenstrom 1997a
-
- Wenstrom KD, Weiner CP, Merrill D, Niebyl J. A placebo‐controlled randomized trial of the terbutaline pump for prevention of preterm delivery. American Journal of Perinatology 1997;14(2):87‐91. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

