Recent advances on plasmin inhibitors for the treatment of fibrinolysis-related disorders
- PMID: 24659483
- PMCID: PMC8788159
- DOI: 10.1002/med.21315
Recent advances on plasmin inhibitors for the treatment of fibrinolysis-related disorders
Abstract
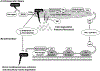
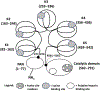
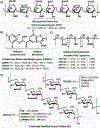
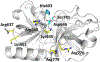
Growing evidence suggests that plasmin is involved in a number of physiological processes in addition to its key role in fibrin cleavage. Plasmin inhibition is critical in preventing adverse consequences arising from plasmin overactivity, e.g., blood loss that may follow cardiac surgery. Aprotinin was widely used as an antifibrinolytic drug before its discontinuation in 2008. Tranexamic acid and ε-aminocaproic acid, two small molecule plasmin inhibitors, are currently used in the clinic. Several molecules have been designed utilizing covalent, but reversible, chemistry relying on reactive cyclohexanones, nitrile warheads, and reactive aldehyde peptidomimetics. Other major classes of plasmin inhibitors include the cyclic peptidomimetics and polypeptides of the Kunitz and Kazal-type. Allosteric inhibitors of plasmin have also been designed including small molecule lysine analogs that bind to plasmin's kringle domain(s) and sulfated glycosaminoglycan mimetics that bind to plasmin's catalytic domain. Plasmin inhibitors have also been explored for resolving other disease states including cell metastasis, cell proliferation, angiogenesis, and embryo implantation. This review highlights functional and structural aspects of plasmin inhibitors with the goal of advancing their design.
Keywords: allosteric inhibition; aprotinin; cyclic peptidomimetics; glycosaminoglycan mimetics; plasmin(ogen); serine proteases antifibrinolytics; tranexamic acid.
© 2014 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
Authors declare no competing financial conflict of interest.
Figures
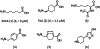


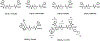




References
-
- Levy JH. Pharmacologic methods to reduce perioperative bleeding. Transfusion 2008;48:31S–38S. - PubMed
-
- Dhir A Antifibrinolytics in cardiac surgery. Ann Card Anaesth 2013;16:117–125. - PubMed
-
- Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50:753–765. - PubMed
-
- Trudell J, McMurdy N. Current antifibrinolytic therapy for coronary artery revascularization. AANA 2008;76:121–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

