The blood exposome and its role in discovering causes of disease
- PMID: 24659601
- PMCID: PMC4123034
- DOI: 10.1289/ehp.1308015
The blood exposome and its role in discovering causes of disease
Abstract
Background: Since 2001, researchers have examined the human genome (G) mainly to discover causes of disease, despite evidence that G explains relatively little risk. We posit that unexplained disease risks are caused by the exposome (E; representing all exposures) and G × E interactions. Thus, etiologic research has been hampered by scientists' continuing reliance on low-tech methods to characterize E compared with high-tech omics for characterizing G.
Objectives: Because exposures are inherently chemical in nature and arise from both endogenous and exogenous sources, blood specimens can be used to characterize exposomes. To explore the "blood exposome" and its connection to disease, we sought human blood concentrations of many chemicals, along with their sources, evidence of chronic-disease risks, and numbers of metabolic pathways.
Methods: From the literature we obtained human blood concentrations of 1,561 small molecules and metals derived from foods, drugs, pollutants, and endogenous processes. We mapped chemical similarities after weighting by blood concentrations, disease-risk citations, and numbers of human metabolic pathways.
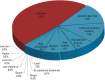
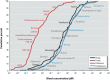
Results: Blood concentrations spanned 11 orders of magnitude and were indistinguishable for endogenous and food chemicals and drugs, whereas those of pollutants were 1,000 times lower. Chemical similarities mapped by disease risks were equally distributed by source categories, but those mapped by metabolic pathways were dominated by endogenous molecules and essential nutrients.
Conclusions: For studies of disease etiology, the complexity of human exposures motivates characterization of the blood exposome, which includes all biologically active chemicals. Because most small molecules in blood are not human metabolites, investigations of causal pathways should expand beyond the endogenous metabolome.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
