The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells
- PMID: 24659632
- PMCID: PMC4047499
- DOI: 10.1182/blood-2013-10-535112
The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells
Abstract
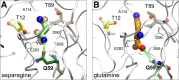
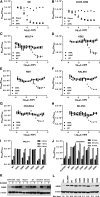
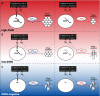
L-Asparaginase (L-ASP) is a key component of therapy for acute lymphoblastic leukemia. Its mechanism of action, however, is still poorly understood, in part because of its dual asparaginase and glutaminase activities. Here, we show that L-ASP's glutaminase activity is not always required for the enzyme's anticancer effect. We first used molecular dynamics simulations of the clinically standard Escherichia coli L-ASP to predict what mutated forms could be engineered to retain activity against asparagine but not glutamine. Dynamic mapping of enzyme substrate contacts identified Q59 as a promising mutagenesis target for that purpose. Saturation mutagenesis followed by enzymatic screening identified Q59L as a variant that retains asparaginase activity but shows undetectable glutaminase activity. Unlike wild-type L-ASP, Q59L is inactive against cancer cells that express measurable asparagine synthetase (ASNS). Q59L is potently active, however, against ASNS-negative cells. Those observations indicate that the glutaminase activity of L-ASP is necessary for anticancer activity against ASNS-positive cell types but not ASNS-negative cell types. Because the clinical toxicity of L-ASP is thought to stem from its glutaminase activity, these findings suggest the hypothesis that glutaminase-negative variants of L-ASP would provide larger therapeutic indices than wild-type L-ASP for ASNS-negative cancers.
© 2014 by The American Society of Hematology.
Figures



Comment in
-
Is glutamine depletion needed in ALL disease?Blood. 2014 Jun 5;123(23):3532-3. doi: 10.1182/blood-2014-04-565523. Blood. 2014. PMID: 24904098 No abstract available.
Similar articles
-
Glutaminase Activity of L-Asparaginase Contributes to Durable Preclinical Activity against Acute Lymphoblastic Leukemia.Mol Cancer Ther. 2019 Sep;18(9):1587-1592. doi: 10.1158/1535-7163.MCT-18-1329. Epub 2019 Jun 17. Mol Cancer Ther. 2019. PMID: 31209181 Free PMC article.
-
Novel mutant of Escherichia coli asparaginase II to reduction of the glutaminase activity in treatment of acute lymphocytic leukemia by molecular dynamics simulations and QM-MM studies.Med Hypotheses. 2018 Mar;112:7-17. doi: 10.1016/j.mehy.2018.01.004. Epub 2018 Jan 30. Med Hypotheses. 2018. PMID: 29447943
-
In silico engineering of L-asparaginase to have reduced glutaminase side activity for effective treatment of acute lymphoblastic leukemia.J Pediatr Hematol Oncol. 2011 Dec;33(8):617-21. doi: 10.1097/MPH.0b013e31822aa4ec. J Pediatr Hematol Oncol. 2011. PMID: 22042278
-
L-Asparaginase delivery systems targeted to minimize its side-effects.Adv Colloid Interface Sci. 2023 Jun;316:102915. doi: 10.1016/j.cis.2023.102915. Epub 2023 May 3. Adv Colloid Interface Sci. 2023. PMID: 37159987 Review.
-
Circumventing the side effects of L-asparaginase.Biomed Pharmacother. 2021 Jul;139:111616. doi: 10.1016/j.biopha.2021.111616. Epub 2021 Apr 28. Biomed Pharmacother. 2021. PMID: 33932739 Review.
Cited by
-
Molecular Analysis of L-Asparaginases for Clarification of the Mechanism of Action and Optimization of Pharmacological Functions.Pharmaceutics. 2022 Mar 9;14(3):599. doi: 10.3390/pharmaceutics14030599. Pharmaceutics. 2022. PMID: 35335974 Free PMC article. Review.
-
Emerging Therapies for Acute Myelogenus Leukemia Patients Targeting Apoptosis and Mitochondrial Metabolism.Cancers (Basel). 2019 Feb 22;11(2):260. doi: 10.3390/cancers11020260. Cancers (Basel). 2019. PMID: 30813354 Free PMC article. Review.
-
Identification and structural analysis of an L-asparaginase enzyme from guinea pig with putative tumor cell killing properties.J Biol Chem. 2014 Nov 28;289(48):33175-86. doi: 10.1074/jbc.M114.609552. Epub 2014 Oct 15. J Biol Chem. 2014. PMID: 25320094 Free PMC article.
-
A clinically attainable dose of L-asparaginase targets glutamine addiction in lymphoid cell lines.Cancer Sci. 2015 Nov;106(11):1534-43. doi: 10.1111/cas.12807. Epub 2015 Oct 16. Cancer Sci. 2015. PMID: 26331698 Free PMC article.
-
Species-Specificity of Transcriptional Regulation and the Response to Lipopolysaccharide in Mammalian Macrophages.Front Cell Dev Biol. 2020 Jul 21;8:661. doi: 10.3389/fcell.2020.00661. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32793601 Free PMC article.
References
-
- Ortega JA, Nesbit ME, Jr, Donaldson MH, et al. L-Asparaginase, vincristine, and prednisone for induction of first remission in acute lymphocytic leukemia. Cancer Res. 1977;37(2):535–540. - PubMed
-
- Lorenzi PL, Landowski CP, Brancale A, et al. N-methylpurine DNA glycosylase and 8-oxoguanine dna glycosylase metabolize the antiviral nucleoside 2-bromo-5,6-dichloro-1-(beta-D-ribofuranosyl)benzimidazole. Drug Metab Dispos. 2006;34(6):1070–1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

