Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial
- PMID: 24659797
- PMCID: PMC4215282
- DOI: 10.1136/jnnp-2013-307282
Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial
Abstract
Objective: This double-blind, placebo-controlled, dose-finding phase IIb study evaluated the efficacy and safety of ponesimod, an oral selective S1P1 receptor modulator, for the treatment of patients with relapsing-remitting multiple sclerosis (RRMS).
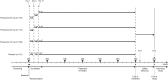
Methods: 464 patients were randomised to receive once-daily oral ponesimod 10, 20 or 40 mg, or placebo for 24 weeks. The primary endpoint was the cumulative number of new T1 gadolinium-enhanced (T1 Gd+) lesions per patient recorded every 4 weeks from weeks 12 to 24 after study drug initiation. Secondary endpoints were the annualised confirmed relapse rate (ARR) and time to first confirmed relapse. Safety and tolerability were also evaluated.
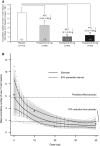
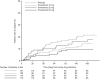
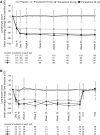
Results: The mean cumulative number of new T1 Gd+ lesions at weeks 12-24 was significantly lower in the ponesimod 10 mg (3.5; rate ratio (RR) 0.57; p=0.0318), 20 mg (1.1; RR 0.17; p<0.0001) and 40 mg (1.4; RR 0.23; p<0.0001) groups compared with placebo (6.2). The mean ARR was lower with 40 mg ponesimod versus placebo, with a maximum reduction of 52% (0.25 vs 0.53; p=0.0363). The time to first confirmed relapse was increased with ponesimod compared with placebo. The proportion of patients with ≥ 1 treatment-emergent adverse events (AEs) was similar across ponesimod groups and the placebo group. Frequently reported AEs with higher incidence in the three ponesimod groups compared with placebo were anxiety, dizziness, dyspnoea, increased alanine aminotransferase, influenza, insomnia and peripheral oedema.
Conclusions: Once-daily treatment with ponesimod 10, 20 or 40 mg significantly reduced the number of new T1 Gd+ lesions and showed a beneficial effect on clinical endpoints. Ponesimod was generally well tolerated, and further investigation of ponesimod for the treatment of RRMS is under consideration.
Trial registration number: NCT01006265.
Keywords: Multiple Sclerosis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures

Comment in
-
Are more sphingosine 1-phosphate receptor agonists a better therapeutic option against multiple sclerosis?J Neurol Neurosurg Psychiatry. 2014 Nov;85(11):1180. doi: 10.1136/jnnp-2013-307538. Epub 2014 Mar 21. J Neurol Neurosurg Psychiatry. 2014. PMID: 24659798 No abstract available.
References
-
- Compston A, Coles A. Multiple sclerosis. Lancet 2008;372:1502–17. - PubMed
-
- Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci 2001;22:117–39. - PubMed
-
- Devonshire V, Lapierre Y, Macdonell R, et al. The Global Adherence Project (GAP): a multicenter observational study on adherence to disease-modifying therapies in patients with relapsing-remitting multiple sclerosis. Eur J Neurol 2011;18:69–77. - PubMed
-
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–15. - PubMed
-
- Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
