Imaging of cerebrovascular pathology in animal models of Alzheimer's disease
- PMID: 24659966
- PMCID: PMC3952109
- DOI: 10.3389/fnagi.2014.00032
Imaging of cerebrovascular pathology in animal models of Alzheimer's disease
Abstract
In Alzheimer's disease (AD), vascular pathology may interact with neurodegeneration and thus aggravate cognitive decline. As the relationship between these two processes is poorly understood, research has been increasingly focused on understanding the link between cerebrovascular alterations and AD. This has at last been spurred by the engineering of transgenic animals, which display pathological features of AD and develop cerebral amyloid angiopathy to various degrees. Transgenic models are versatile for investigating the role of amyloid deposition and vascular dysfunction, and for evaluating novel therapeutic concepts. In addition, research has benefited from the development of novel imaging techniques, which are capable of characterizing vascular pathology in vivo. They provide vascular structural read-outs and have the ability to assess the functional consequences of vascular dysfunction as well as to visualize and monitor the molecular processes underlying these pathological alterations. This article focusses on recent in vivo small animal imaging studies addressing vascular aspects related to AD. With the technical advances of imaging modalities such as magnetic resonance, nuclear and microscopic imaging, molecular, functional and structural information related to vascular pathology can now be visualized in vivo in small rodents. Imaging vascular and parenchymal amyloid-β (Aβ) deposition as well as Aβ transport pathways have been shown to be useful to characterize their dynamics and to elucidate their role in the development of cerebral amyloid angiopathy and AD. Structural and functional imaging read-outs have been employed to describe the deleterious affects of Aβ on vessel morphology, hemodynamics and vascular integrity. More recent imaging studies have also addressed how inflammatory processes partake in the pathogenesis of the disease. Moreover, imaging can be pivotal in the search for novel therapies targeting the vasculature.
Keywords: Alzheimer's disease (AD); amyloid-beta (Aβ); angiography; cerebral amyloid angiopathy (CAA); cerebral blood flow (CBF); magnetic resonance imaging (MRI); microscopy; transgenic mice.
Figures




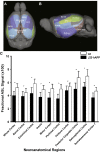

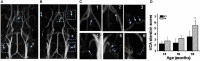


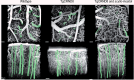

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

