Electrically facilitated translocation of protein through solid nanopore
- PMID: 24661490
- PMCID: PMC3976542
- DOI: 10.1186/1556-276X-9-140
Electrically facilitated translocation of protein through solid nanopore
Abstract
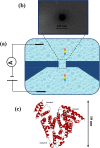
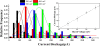
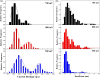
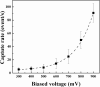
Nanopores have been proven as versatile single-molecule sensors for individual unlabeled biopolymer detection and characterization. In the present work, a relative large nanopore with a diameter of about 60 nm has been used to detect protein translocation driven by a series of applied voltages. Compared with previous studied small nanopores, a distinct profile of protein translocation through a larger nanopore has been characterized. First, a higher threshold voltage is required to drive proteins into the large nanopore. With the increase of voltages, the capture frequency of protein into the nanopore has been markedly enhanced. And the distribution of current blockage events is characterized as a function of biased voltages. Due to the large dimension of the nanopore, the adsorption and desorption phenomenon of proteins observed with a prolonged dwell time has been weakened in our work. Nevertheless, the protein can still be stretched into an unfolded state by increased electric forces at high voltages. In consideration of the high throughput of the large nanopore, a couple of proteins passing through the nanopore simultaneously occur at high voltage. As a new feature, the feasibility and specificity of a nanopore with distinct geometry have been demonstrated for sensing protein translocation, which broadly expand the application of nanopore devices.
Figures




Similar articles
-
Voltage-driven translocation of DNA through a high throughput conical solid-state nanopore.PLoS One. 2012;7(9):e46014. doi: 10.1371/journal.pone.0046014. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029365 Free PMC article.
-
Biological Nanopores: Confined Spaces for Electrochemical Single-Molecule Analysis.Acc Chem Res. 2018 Feb 20;51(2):331-341. doi: 10.1021/acs.accounts.7b00143. Epub 2018 Jan 24. Acc Chem Res. 2018. PMID: 29364650
-
Detection and Separation of DNA and Silver Nanoparticles Using a Solid-State Nanopore.ACS Omega. 2023 May 9;8(20):17682-17688. doi: 10.1021/acsomega.3c00152. eCollection 2023 May 23. ACS Omega. 2023. PMID: 37251189 Free PMC article.
-
Plasmonic-Nanopore Biosensors for Superior Single-Molecule Detection.Adv Mater. 2019 Jun;31(23):e1900422. doi: 10.1002/adma.201900422. Epub 2019 Apr 3. Adv Mater. 2019. PMID: 30941823 Review.
-
Characterization of protein unfolding with solid-state nanopores.Protein Pept Lett. 2014 Mar;21(3):256-65. doi: 10.2174/09298665113209990077. Protein Pept Lett. 2014. PMID: 24370259 Free PMC article. Review.
Cited by
-
Hydrogen Peroxide Sensing Based on Inner Surfaces Modification of Solid-State Nanopore.Nanoscale Res Lett. 2017 Dec;12(1):422. doi: 10.1186/s11671-017-2190-x. Epub 2017 Jun 20. Nanoscale Res Lett. 2017. PMID: 28637348 Free PMC article.
-
Optofluidic devices with integrated solid-state nanopores.Mikrochim Acta. 2016 Apr;183(4):1275-1287. doi: 10.1007/s00604-016-1758-y. Epub 2016 Jan 27. Mikrochim Acta. 2016. PMID: 27046940 Free PMC article.
-
DNA-functionalized silicon nitride nanopores for sequence-specific recognition of DNA biosensor.Nanoscale Res Lett. 2015 May 1;10:205. doi: 10.1186/s11671-015-0909-0. eCollection 2015. Nanoscale Res Lett. 2015. PMID: 25977675 Free PMC article.
-
Single Nanoparticle Translocation Through Chemically Modified Solid Nanopore.Nanoscale Res Lett. 2016 Dec;11(1):50. doi: 10.1186/s11671-016-1255-6. Epub 2016 Feb 1. Nanoscale Res Lett. 2016. PMID: 26831688 Free PMC article.
-
Sensing Native Protein Solution Structures Using a Solid-state Nanopore: Unraveling the States of VEGF.Sci Rep. 2018 Jan 17;8(1):1017. doi: 10.1038/s41598-018-19332-y. Sci Rep. 2018. PMID: 29343861 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

